Abstract
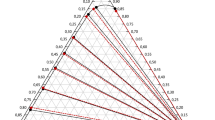
Liquid–liquid equilibrium (LLE) data for the methyl isobutyl ketone + o-cresol + water ternary system have been experimentally measured from 298.2 to 323.2 K below 101 kPa. The measured LLE data were verified to be highly consistent through the Othmer–Tobias and Hand equations. The extraction efficiency of methyl isobutyl ketone for o-cresol was assessed by the distribution coefficient and separation factor. The experimental results correlated well with two excess Gibbs energy models: non-random two-liquid and universal quasi chemical, which also yielded binary interaction parameter.






Similar content being viewed by others
References
Ji, Q.H., Tabassum, S., Hena, S., Silva, C.G., Yu, G.X., Zhang, Z.J.: A review on the coal gasification wastewater treatment technologies: past, present and future outlook. J. Clean. Prod. 126, 38–55 (2016)
Helmes, C.T., Levin, B., McCaleb, K.: US Environmental Protection Agency, Washington DC (1978)
Koch, R.: Umweltchemikalien, 3rd edn., pp. 284–287. VCH Verlagsgesellschaft mbH, Weinheim (1995)
Dietz, F., Traud, J.: Geruchs- und geschmacks-schwellen-konzentrationen von phenolkorpern. Gas- und Wasserfach (GWF): Wasser/Ab-Wasser 119, 318–325 (1978)
Toxic substances control act (TSCA) chemical substance inventory. US Environmental Protection Agency (EPA), Washington DC (1979)
Chen, Y., Wang, H.M., Lv, R., Li, L.B.: Phase equilibrium measurement and thermodynamic modeling of the 4-methylpetan-2-one/3-methylphenol, and 4-methylphenol/water ternary systems at 298.2, 313.2, and 323.2 K and 0.1 MPa. J. Chem. Eng. Data 62, 141–147 (2017)
Luo, L.J., Liu, D., Li, L.B., Chen, Y.: Measurements and thermodynamic modeling of liquid–liquid equilibria in ternary system 2-methoxy-2-methylpropane + p-cresol + water. Chin. J. Chem. Eng. 24, 360–364 (2016)
Lu, H.J., Wang, J.K., Yu, J., Wu, Y.F., Wang, T., Bao, Y., Ma, D., Hao, H.X.: Phase equilibria for the pseudo-ternary system (NaCl + Na2SO4 + H2O) of coal gasification wastewater at T = (268.15 to 373.15) K. Chin. J. Chem. Eng. (2016). https://doi.org/10.1016/j.cjche.2016.08.016
Luo, L.J., Liu, D., Li, L.B., Chen, Y.: Experimental determination and correlation of liquid liquid equilibria for the ternary system 2-methoxy-2-methylpropane–o-cresol–water. J. Chem. Eng. Data 60, 1396–1400 (2015)
Yu, Z.J., Chen, Y., Feng, D.C., Qian, Y.: Process development, simulation and industrial implementation of new coal-gasification wastewater treatment installation for phenol and ammonia removal. Ind. Eng. Chem. Res. 49, 2874–2881 (2010)
Feng, D.C., Yu, Z.J., Chen, Y., Qian, Y.: Novel single stripper with side-draw to remove ammonia and sour gas simultaneously for coal-gasification wastewater treatment and the industrial implementation. Ind. Eng. Chem. Res. 48, 5816–5823 (2009)
Yang, C.F., Qian, Y., Zhang, L.J., Feng, J.Z.: Solvent extraction process development and on-site trial-plant for phenol removal from industrial coal-gasification wastewater. Chem. Eng. J. 117, 179–185 (2006)
Yang, C.F., Qian, Y., Zhang, L.J., Feng, J.Z.: Measurement and correlation of liquid–liquid equilibrium data for methyl isobutyle ketone–water–phenol ternary system. J. Chem. Ind. Eng. (China) 58, 805–809 (2007)
Yang, C.F., Qian, Y., Guo, J.W., Chen, J.R.: Liquid–liquid equilibria for the ternary system methyl isobutyl ketone + 1,2-benzenediol + water. J. Chem. Eng. Data 59, 3663–3667 (2014)
Yang, C.F., Qian, Y., Guo, J.W., Chen, J.R., Peng, J.P.: Liquid–liquid equilibria for the ternary system methyl isobutyl ketone + 1,2-benzenediol + water. J. Chem. Eng. Data 58, 3324–3328 (2014)
Yang, C.F., Jiang, Y.B., Zhang, L.J., Qian, Y.: Liquid–liquid equilibria for the ternary system methyl isobutyl ketone + water + hydroquinone. J. Chem. Eng. Data 51, 2107–2109 (2006)
Yang, C.F., Qian, Y., Jiang, Y.B., Zhang, L.J.: Liquid–liquid equilibria for the quaternary system methyl isobutyl ketone–water–phenol–hydroquinone. Fluid Phase Equilib. 258, 73–77 (2007)
Fang, W.W., Liu, G.Z., Wang, L., Zhang, X.W.: Liquid–liquid equilibria for the ternary system water + methyl isobutyl ketone + tert-butyl alcohol at several temperatures. J. Chem. Eng. Data 53, 466–470 (2008)
Zhang, L., Zhang, H.X., Chen, L.: Measurement of liquid–liquid equilibrium data for water–acrylic acid–methyl isobutyl ketone. Zhengzhou Daxue Xuebao 32, 46–48 (2011)
Renon, H., Prausnitz, J.M.: Local compositions in thermodynamic excess functions for liquid mixtures. AIChE J. 14, 135–144 (1968)
Abrams, D.S., Prausnitz, J.M.: Statistical thermodynamics of liquid mixtures: a new expression for the excess Gibbs energy of partly or completely miscible systems. AIChE J. 21, 116–128 (1975)
Hand, D., Distribution, D.: Dineric distribution. J. Phys. Chem. 34, 1961–2000 (1929)
Othmer, D., Tobias, P.: Liquid–liquid extraction data–tie line correlation. Ind. Eng. Chem. Res. 34, 693–696 (1942)
Lv, R., Wang, Z., Li, L., Chen, Y.: Liquid–liquid equilibria in the ternary systems water + cresols + methyl butyl ketone at 298.2 and 313.2 K: experimental data and correlation. Fluid Phase Equilib. 404, 89–95 (2015)
Zhou, S., Liao, M., Liu, D., Li, L., Chen, Y.: Liquid–liquid equilibrium for the ternary systems methyl tert-butyl ketone + o-, m-, p-cresol + water at (298.2, 313.2 and 323.2) K. J. Chem. Eng. Data 62, 1929–1936 (2017)
Mafra, M.R., Krähenbühl, M.A.: Liquid–liquid equilibrium of (water + acetone) with cumene or α-methylstyrene or phenol at temperatures of (323.15 and 333.15) K. J. Chem. Eng. Data 51, 753–756 (2006)
Magnussen, T., Rasmussen, P., Fredenslund, A.: UNIFAC parameter table for prediction of liquid–liquid equilibria. Ind. Eng. Chem. Process. Des. Dev. 20, 331–339 (1981)
Acknowledgments
Financial support from the National Science Foundation of China (21506066), State Key Laboratory of Pulp and Paper Engineering (201708), the Guangzhou Technology Project (20181002SF0525), and the Fundamental Research Funds for the Central Universities SCUT (2017ZD069) are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, Y., Xiong, K., Zhou, S. et al. Liquid–Liquid Equilibrium of Ternary Systems of Methyl Isobutyl Ketone + o-Cresol + Water at 298.2, 313.2 and 323.2 K. J Solution Chem 46, 2204–2213 (2017). https://doi.org/10.1007/s10953-017-0698-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-017-0698-4