Abstract
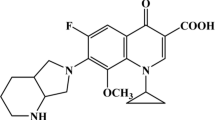
Potentiometric measurements of the interaction of sparfloxacin (SPFX) and metal ions Co(II), Ni(II), Cu(II), Zn(II) and Cd(II) with nucleosides (NS) adenosine, guanosine, cytidine and inosine were carried out. The complexes formed are monoprotonated M(II)(SPFX)(HNS) species, where the nucleoside acts as a secondary ligand in its protonated form. The formation of ternary complexes of some systems was confirmed by UV–Visible measurements in solution. The binding constant of M(II)–SPFX complexes were measured in Tris–HCl buffer solution (pH = 7.4).









Similar content being viewed by others
References
Pillai, S.I., Vijayaraghavan, K., Subramanian, S.: Evaluation of DNA-binding, cleavage, BSA interaction of Zn-hydroxy flavone complex. Der. Pharma. Chemica 6, 379–389 (2014)
Chen, D., Milacic, V., Frezza, M., Dou, Q.P.: Metal complexes, their cellular targets and potential for cancer therapy. Curr. Pharm. Des. 15, 777–791 (2009)
Fricker, S.P.: Metal based drugs: from serendipity to design. Dalton Trans. 43, 4903–4917 (2007)
Meggers, E.: Targeting proteins with metal complexes. Chem. Commun. 9, 1001–1010 (2009)
Yan, Y.K., Melchart, M., Habtemariam, A., Sadler, P.J.: Organometallic chemistry, biology and medicine: ruthenium arene anticancer complexes. Chem. Commun. 38, 4764–4776 (2005)
Cohen, S.M.: New approaches for medicinal applications of bioinorganic chemistry. Curr. Opin. Chem. Biol. 11, 115–120 (2007)
Ott, I., Gust, R.: Non platinum metal complexes as anti-cancer drugs. Arch. Pharm. (Weinheim) 340, 117–126 (2007)
Hambley, T.W.: Developing new metal based therapeutics: challenges and opportunities. Dalton Trans. 43, 4929–4937 (2007)
Odani, A., Jastrzab, R., Lomozik, L.: Equilibrium study on the interaction of phytic acid with polyamines and metal ions. Metallomics 73, 735–743 (2011)
Orvig, C., Abrams, M.J.: Medicinal inorganic chemistry: introduction. Chem. Rev. 99, 2201–2204 (1999)
Thompson, K.H., Orvig, C.: Boon and bane of metal ions in medicine. Science 300, 936–939 (2003)
Haas, K.L., Franz, K.J.: Application of metal coordination chemistry to explore and manipulate cell biology. Chem. Rev. 109, 4921–4960 (2009)
Al-Mustafa, J., Shinar, A.: Conductometric determination of the stability constants of the quinolone antibacterial agents levofloxacin and sparfloxacin with divalent metal ions. Jordan J. Chem. 8, 237–246 (2013)
Azab, H.A., Anwar, Z.M., Al-Deyab, S.S., Kamel, R.M.: Potentiometric, electrochemical, and fluorescence study of the coordination properties of the monomeric and dimeric complexes of Eu(III) with nucleobases and PIPES. J. Chem. Eng. Data 56, 1960–1969 (2011)
Azab, H.A., Anwar, Z.M., Al-Deyab, S.S., Abd El-Gawad, I.I., Kamel, R.M.: Comparison of the coordination tendency of amino acids, nucleobases, or mononucleotides toward the monomeric and dimeric lanthanide complexes with biologically important compounds. J. Chem. Eng. Data 56, 2613–2625 (2011)
Azab, H.A., Abd El-Gawad, I.I., Kamel, R.M.: Ternary complexes formed by the fluorescent probe Eu(III)-9-anthracene carboxylic acid with pyrimidine and purine nucleobases. J. Chem. Eng. Data 54, 3069–3078 (2009)
Azab, H.A., Anwar, Z.M., Kamel, R.M.: Ternary complexes formed among Pd(II) ions nucleobases, nucleosides, nucleotides and some biologically important compounds. J. Solution Chem. 45, 1095–1114 (2016)
Welcher, F.J.: The Analytical Uses of Ethylene Diaminetetraacetic Acid. D.Von Nostrand Co., Inc., Princeton (1965)
Lawrence, D., Vaidyanathan, V.G., Unni Nair, B.: Synthesis, characterization and DNA binding studies of two mixed ligand complexes of ruthenium(II). J. Inorg. Biochem. 100, 1244–1251 (2006)
Eshkourfu, R., Čobeljić, B., Vujčić, M., Turel, I., Pevec, A., Sepčić, K., Zec, M., Radulović, S., Srdić-Radić, T., Mitić, D., Andjelković, K., Sladić, D.: Synthesis, characterization, cytotoxic activity and DNA binding properties of the novel dinuclear cobalt(III) complex with the condensation product of 2-acetylpyridine and malonic acid dihydrazide. J. Inorg. Biochem. 105, 1196–1203 (2011)
Bates, R.B., Paabo, M., Robinson, R.A.: Interpretation of pH measurements in alcohol–water solvents. J. Phys. Chem. 67, 1833–1845 (1963)
Gran, G.: Determination of the equivalence point in potentiometric titration. Analyst 77, 661–671 (1952)
De Stefano, C., Princi, P., Rigano, C., Sammartono, S.: Computer analysis of equilibrium data in solution. ESAB2M: an improved version of the ESAB program. Ann. Chim (Rome) 77, 643–675 (1987)
Gans, P., Sabatini, A., Vacca, A.: Investigation of equilibria in solution. Determination of equilibrium constants with the HYPERQUAD suite of programs. Talanta 43, 1739–1753 (1996)
Bologni, L., Sabatini, A., Vacca, A.: Complex formation equilibria between 2-amino-2(hydroxymethyl)-1,3,-propanediol (tris, tham) and nickel(II), copper(II), zinc(II) and hydrogen ions in aqueous solutions. Inorg. Chim. Acta 69, 71–75 (1983)
Wolfe, A., Shimer, G.H., Meehan, T.: Polycyclic aromatic hydrocarbons physically intercalate into duplex regions of denatured DNA. Biochemistry 26, 6392–6396 (1987)
Stephanos, J.J.: Drug–protein interactions: Two-site binding of heterocyclic ligands to a monomeric hemoglobin. J. Inorg. Biochem. 62, 155–169 (1996)
Dahloff, A.: Quinolones in antibiotics and chemotherapy. In: Shorfeldd, H. (ed.) Pharmacokinetics of Selected Antibacterial Agents, pp. 85–108. Karger, New York (1998)
Sigel, H.: Metal Ions in Biological Systems. Marcel Dekker Inc., New York (1979)
Lippared, S.J.: Progress in Inorganic Chemistry, vol. 37, pp. 214–215. Wiley (1989)
Smith, R.M., Martell, A.E., Chen, Y.: Critical evaluation of stability constants for nucleotide complexes with protons and metal ions and the accompanying enthalpy changes. Pure Appl. Chem. 63, 1015–1080 (1991)
Connors, T.A., Roberts, J.J.: Platinum Coordination Complexes in Cancer Chemotherapy. Springer, New York (1974)
Martin, R.B., Mariam, Y.H.: Metal Ions in Biological Systems, vol. 8, pp. 57–124. Marcel Dekker, New York (1979)
Martin, R.B.: Nucleoside sites for transition metal ion binding. Acct. Chem. Res. 18, 32–38 (1985)
Hodgson, D.J.: The stereochemistry of metal complexes of nucleic acid constituents. Prog. Inorg. Chem. 23, 211–254 (1977)
Urbaniak, B., Kokot, Z.J.: Spectroscopic investigations of fluoroquinolones metal ion complexes. Acta Pol. Pharm. 70, 621–629 (2013)
Ploeser, J.M., Loring, H.S.: The Ultraviolet absorption spectra of the pyrimidine ribonucleosides and ribonucleotides. J. Biol. Chem. 178, 431–437 (1949)
Chaveerach, U., Meenongwa, A., Trongpanich, Y., Soikum, C., Chaveerach, P.: DNA binding and cleavage behaviors of copper(II) complexes with amidino-O-methylurea and N-methylphenyl-amidino-O-methylurea, and their antibacterial activities. Polyhedron 29, 731–738 (2010)
Anupama, B., Aruna, A., Jhansi Lakshmi, P., Swapna, V.: Synthesis, characterization of metal complexes with anthranilic acid based Schiff base: DNA binding, cleavage, antimicrobial, antioxidative and BSA binding studies. Int. J. Inorg. Bioinorg. Chem. 6, 11–22 (2016)
Satyanarayana, S., Dabrowiak, J.C., Chaires, J.B.: Neither Δ- nor Λ-tris(phenanthroline) ruthenium(II) binds to DNA by classical intercalation. Biochemistry 31, 9319–9324 (1992)
Chaires, J.B., Dattagupta, N., Crothers, D.M.: Studies on interaction of anthracycline antibiotics and deoxyribonucleic acid: equilibrium binding studies on the interaction of daunomycin with deoxyribonucleic acid. Biochemistry 21, 3933–3940 (1982)
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Kamel, R.M., Hegazy, W.H., Ali, R.S. et al. Sparfloxacin Metal Complexes with Nucleosides: Potentiometric and Spectral Studies. J Solution Chem 46, 1680–1697 (2017). https://doi.org/10.1007/s10953-017-0669-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-017-0669-9