Abstract
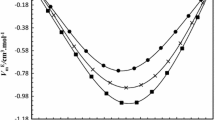
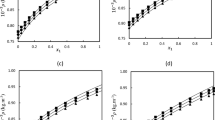
2-Methylpropan-2-ol, an important fine chemical, may be dehydrated during extractive distillation with glycols as entrainer. Experimental isobaric phase equilibrium studies were carried out on binary mixtures of 2-methylpropan-2-ol with ethane-1,2-diol, as an entrainer, at the local atmospheric pressure of 94.99 kPa and at sub-atmospheric pressures of 19.99, 39.99, 59.99, 78.79 kPa using a Sweitoslawski-type ebulliometer. The Wilson and NRTL activity coefficient models were used to correlate the experimental results and the binary interaction parameters were obtained using the Generalized Reduced Gradient optimization technique. UNIFAC was also used to predict the deviations in bubble temperatures. Moreover, the variation in density, refractive index values and other derived properties (excess molar volumes, partial molar volumes and deviations in molar refractivity) were explored at 303.15, 313.15, 323.15 and 333.15 K to understand the shift of equilibrium with the variation in the mixture composition for the conformational state of the molecules. The Redlich–Kister polynomial equation was used to correlate the excess molar volumes and deviations from molar refractivity. Different theoretical mixing rules (Lorentz–Lorenz, Wiener, Heller, Gladstone–Dale and Arago–Biot) are investigated and reported in terms of average percentage deviation. Furthermore, the Prigogine–Flory–Patterson theory was used to predict the trend of the dependence of excess molar volumes on composition for the present system.






Similar content being viewed by others
References
Aniya, V., Muktham, R.K., Kumari, A., Satyavathi, B.: Modeling and simulation of batch kinetics of non-edible Karanja oil for biodiesel production: a mass transfer study. Fuel 161, 137–145 (2015)
Frusteri, F., Arena, F., Bonura, G., Cannilla, C., Spadaro, L., Blasi, O.D.: Catalytic etherification of glycerol by tert-butyl alcohol to produce oxygenated additives for diesel fuel. Appl. Catal. A: Gen. 367, 77–83 (2009)
Hwang, I.C., Kwon, R.H., Park, S.J.: Azeotrope breaking for the system ethyl tert-butyl ether (ETBE) + ethanol at 313.15 K and excess properties at 298.15 K for mixtures of ETBE and ethanol with phosphonium-based ionic liquids. Fluid Phase Equilib. 344, 32–37 (2013)
Aniya, V., De, D., Singh, A., Satyavathi, B.: Isobaric phase equilibrium of tert-butyl alcohol + glycerol at local and subatmospheric pressures, volumetric properties, and molar refractivity from 303.15 to 333.15 K of tert-butyl alcohol + glycerol, tert-butyl alcohol + water, and water + glycerol binary systems. J. Chem. Eng. Data 61, 1850–1863 (2016)
Aniya, V., De, D., Singh, A., Thella, P.K., Satyavathi, B.: Measurement and modelling of solubility of MgCl2 in 2-methylpropan2-ol + water + glycerol + MgCl2 system: a solid–liquid equilibrium study based on symmetric eNRTL model and evaluation of thermodynamic functions of solutions. J. Mol. Liq. 221, 262–270 (2016)
Aniya, V., De, D., Singh, A., Satyavathi, B.: An energy efficient route for the dehydration of 2-methylpropan-2-ol: experimental investigation, modeling and process optimization. Sep. Purif. Technol. 156, 738–753 (2015)
Franco, J.P., Ladosa, E., Loras, S., Monton, J.B.: Volumetric properties of mono-, di-, tri, and polyethylene glycol aqueous solutions from (273.15 to 363.15) K: experimental measurements and correlations. Chem. Eng. Process. 91, 121–129 (2015)
Waheed, A., Breil, M.P., Theveneau, P., Mohammadi, A.H., Kontogeorgis, G.M., Dominique, R.: Phase equilibria of mixtures containing glycol and n-alkane: experimental study of infinite dilution activity coefficients and modeling using the cubic-plus-association equation of state. Ind. Eng. Chem. Res. 48, 11202–11210 (2009)
Wagner, L.R.S., Camila, S.S., Meleiro, L.A.C., Mendes, M.F.: Vapor–liquid equilibrium of the (water + ethanol + glycerol) system: experimental and modelling data at normal pressure. J. Chem. Thermodyn. 67, 106–111 (2013)
Han, K.J., Park, J.H.: densities and refractive indices of the ternary system ethyl tert-butyl ether (ETBE) + ethanol + benzene and its binary sub-systems at 298.15. J. Ind. Eng. Chem. 13, 360–366 (2007)
Mehrdad, M., Ahmad, M., Abdollah, O., Rostami, A.A.: Thermodynamic study on some alkanediol solutions: measurement and modelling. Thermochim. Acta 561, 1–13 (2013)
Kamihama, N., Matsuda, H., Kurihara, K., Tochigi, K., Oba, S.: Isobaric vapor–liquid equilibria for ethanol + water + ethylene glycol and its constituent three binary systems. J. Chem. Eng. Data 57, 339–344 (2012)
Villamanan, M.A., Corlos, G., Van Ness, H.C.: Excess thermodynamic properties for water/ethylene glycol. J. Chem. Eng. Data 29, 427–429 (1984)
Pavel, H., Lubomír, H., Ivan, C.: Partial molar volumes of organic solutes in water. XIV. Polyhydric alcohols derived from ethane and propane at temperatures T = 298 K to T = 573 K and at pressures up to 30 MPa. J. Chem. Thermodyn. 38, 801–809 (2006)
Iloukhani, H., Bahrami, H.: Excess molar volumes and partial molar volumes for binary mixtures of water with 1,2-ethanediol, 1,2-propanediol, and 1,2-butanediol at 293.15, 303.15 and 313.15 K. Phys. Chem. Liq. 38, 103–111 (2000)
Lei, L.H., Duan, Z.T., Xu, Y.F., Qian, W.C., Zhou, R.Q., Ji, S.F.: Study of salt-containing extractive distillation. (II) Development of purificatory process for tert-butanol. Petrochem. Technol. (China) 11, 404–409 (1982)
Wilson, G.M.: Vapor–liquid equilibrium. XI. A new expression for the excess free energy of mixing. J. Am. Chem. Soc. 86, 127–130 (1964)
Renon, H., Prausnitz, J.M.: Local compositions in thermodynamic excess functions for liquid mixtures. AIChE J. 14, 135–144 (1968)
Redlich, O., Kister, A.T.: Algebraic representation of thermodynamic properties and the classification of solutions. Ind. Eng. Chem. 40, 345–348 (1948)
Ciocirlan, O., Iulian, O.: Density, viscosity and refractive index of the dimethyl sulfoxide + o-xylene system. J. Serb. Chem. Soc. 74, 317–329 (2009)
Mehra, R.: Application of refractive index mixing rules in binary systems of hexadecane and heptadecane with n-alkanols at different temperatures. Proc. Indian Acad. Sci. 115, 147–154 (2003)
Tasic, A.Z., Djordjevic, B.D., Grozdanic, D.K., Radojkovic, N.: Use of mixing rules in predicting refractive indexes and specific refractivities for some binary liquid mixtures. J. Chem. Eng. Data 37, 310–313 (1992)
Isehunwa, S.O., Olanisebe, E.B., Ajiboye, O.O., Akintola, S.A.: Estimation of the refractive indices of some binary mixtures. Afr. J. Pure Appl. Chem. 9, 58–64 (2015)
Egorov, G.I., Makarov, D.M.: Densities and volume properties of (water + tert-butanol) over the temperature range of (274.15 to 348.15) K at pressure of 0.1 MPa. J. Chem. Thermodyn. 43, 430–441 (2011)
Kim, E.S., Marsh, K.N.: Excess volumes for 2-methyl-2-propanol—water at 5 K intervals from 303.15 to 323.15 K. J. Chem. Eng. Data 33, 288–292 (1988)
Egorov, G.I., Makarov, D.M.: Volumetric properties of binary mixtures of glycerol + tert-butanol over the temperature range 293.15 to 348.15 K at atmospheric pressure. J. Solution Chem. 4, 554–1536 (2012)
Egorov, G.I., Makarov, D.M.: Volumetric properties of the binary mixture of ethylene glycol + tert-butanol at T = (278.15, 288.15, 298.15, 308.15, 323.15, 333.15, 348.15) K under atmospheric pressure. J. Mol. Liq. 171, 29–36 (2012)
Marchetti, A., Pretl, C., Tagliazucchi, M., Tad, L., Tosi, G.: The N,N-dimethylformamide + ethane-1,2-diol solvent system. dielectric constant, refractive index, and related properties at various temperatures. J. Chem. Eng. Data 36, 365–368 (1991)
Tsierkezos, N.G., Molinou, I.E.: Thermodynamic properties of water + ethylene glycol at 283.15, 293.15, 303.15, and 313.15 K. J. Chem. Eng. Data 43, 989–993 (1998)
Cocchi, M., Manfredini, M., Marchetti, A., Sighinolfi, S., Tassi, L., Ulrici, A., Vignali, M.: The ethane-1,2-D1ol + 2-methoxyethanol + 1,2-D1methoxyethane ternary solvent system: density and volume properties at different temperatures. Phys. Chem. Liq. 39, 481–498 (2001)
Waheed, A., Mohammadi, A.H., Richon, D.: Volumetric properties of mono-, di-, tri-, and polyethylene glycol aqueous solutions from (273.15 to 363.15) K: experimental measurements and correlations. J. Chem. Eng. Data 54, 1254–1261 (2009)
Azizian, S., Bashavard, N.: Surface properties of diluted solutions of cyclohexanol and cyclopentanol in ethylene glycol. J. Colloid Interface Sci. 282, 428–433 (2005)
Hala, E., Pick, J., Fried, V., Villim, O.: Vapor Liquid-Equilibrium. Pergamon Press, Oxford (1958)
Kumari, A., Rane, N.V., Thella, P.K., Satyavathi, B.: Isobaric phase equilibrium at local and sub-atmospheric pressures, molar refractivity, volumetric and viscometric properties of toluene (1) + para-tert-butyltoluene (2) at temperatures (293.15 to 308.15) K: experimental studies and thermodynamic modeling. J. Mol. Liq. 219, 444–462 (2016)
Poling, B.E., Prausnitz J.M., O’Connell, J.P.: The Properties of Gases and Liquids, Fifth edn. McGraw–Hill, New York (2001)
Dean, J.A.: Lange’s Handbook of Chemistry. Fifteenth edn. McGraw–Hill, New York (1999)
Jones, W.S., Tamplin, W.S.: Physical Properties of Ethylene Glycol in Glycols. Curme, G.O. (ed.). Reinhold Publishing Corporation, New York, U.S.A. pp. 27–62 (1952)
Reid, R.C., Prausnitz, J.M., Poling, B.E.: The Properties of Gases and Liquids. McGraw–Hill, New York (1987)
Edgar, T.F., Himmelblau, D.M.: Optimization of Chemical Processes. McGraw–Hill, New York (1988)
Fredenslund, A., Gmehling, J., Rasmussen, P.: A Group Contribution Method. Vapor–Liquid Equilibria Using UNIFAC. Elsevier, Amsterdam (1977)
Hansen, H.K., Rasmussen, P., Fredenslund, A., Schiller, M., Gmehling, J.: Vapor–liquid equilibria by UNIFAC group-contribution. 5. Revision and extension. Ind. Eng. Chem. Res. 30, 2352–2355 (1991)
Fukasawa, T., Tominaga, Y., Wakisaka, A.: Molecular association in binary mixtures of tert-butyl alchol–water and tetrahydrofuran–heavy water studied by mass spectrometry of clusters from liquid droplets. J. Phys. Chem. A 108, 59–63 (2004)
Gubskaya, A.V., Kusalik, P.G.: Molecular dynamics simulation study of ethylene glycol, ethylenediamine, and 2-aminoethanol. 1. The local structure in pure liquids. J. Phys. Chem. A 108, 7151–7164 (2004)
Kumari, A., Aniya, V., Rane, N.V., Thella, P.K., Satyavathi, B.: Isobaric phase equilibrium of morpholine + 1-decanol, volumetric properties and molar refractivity from 293.15 to 333.15 K of morpholine + 1-decanol and 1-octanol + toluene system with applications of Prigogine–Flory–Patterson theory. Thermochim. Acta (2017). doi:10.1016/j.tca.2016.12.010
Aminabhavi, T.M., Golalakrishina, B.: Density, viscosity, refractive index, and speed of sound in aqueous mixtures of N,N-dimethylformamide, dimethyl sulfoxide, N,N-dimethylformamide, acetonitrile, ethylene glycol, diethylene glycol, 1,4-dioxane, tetrahydrofuran, 2-methoxyethanol, and 2-ethoxyethanol at 298.15 K. J. Chem. Eng. Data 40, 856–861 (1995)
Aniya, V., Singh, A., De, D., Reddy, R., Satyavathi, B.: Experimental isobaric vapor–liquid equilibrium at sub-atmospheric and local atmospheric pressures, volumetric properties and molar refractivity from 293.15 to 313.15 K of water + triethlylene glycol. Fluid Phase Equilib. 405, 132–140 (2015)
Kumari, A., Kadakanchi, S., Tella, P.K., Satyavathi, B.: Solubility, Thermodynamics properties and derived excess properties of benzoic acid in (acetic acid + water) and (acetic acid + toulene) binary mixtures. J. Chem. Eng. Data 61, 67–77 (2016)
Rane, N.V., Kumari, A., Soujanya, J., Satyavathi, B.: Excess properties and isobaric vapor–liquid equilibrium at sub-atmospheric pressures of binary (1,2-propanediol + 1,3-propanediol) system: measurement and modelling. J. Chem. Thermodyn. 97, 142–157 (2016)
Aralaguppi, M.I., Jadar, C.V., Aminabhavi, T.M.: Density, refractive index, viscosity, and speed of sound in binary mixtures of cyclohexanone with benzene, methylbenzene, 1,4-dimethylbenzene, 1,3,5-trimethylbenzene, and methoxybenzene in the temperature interval (298.15 to 308.15) K. J. Chem. Eng. Data 44, 446–450 (1999)
Ddamba, W.A.A., Mokoena, T.T., Mokgweetsi, P., Tabbiruka, M.S.N.: Modeling of excess molar volumes of [difurylmethane + (acetonitrile or propionitrile or benzonitrile)] binary mixtures using the Prigogine–Flory–Patterson theory. Am. J. Phys. Chem. 4, 1–5 (2015)
Rodnikovaa, M.N., Troitskiib, V.M., Kayumovaa, D.B., Soloninaa, I.A., Gunina, M.A.: The influence of pressure (0.1–160 MPa) on the isothermal compressibility and bulk viscosity of solutions of tetrahydrofuran in ethylene glycol at 298 K. Russ. J. Phys. Chem. A. 84, 2190–2192 (2010)
Egorov, G.I., Makarov, D.M.: Bulk properties of a liquid phase mixture ethylene glycol + tert-butanol in the temperature range 278.15–348.15 k and pressures of 0.1–100 mPa. ii. Molar isothermal compressibility, molar isobaric expansibility, thermal pressure coefficient, and internal pressure. J. Struct. Chem. 54, 320–335 (2013)
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
10953_2017_638_MOESM1_ESM.docx
Supplementary material 1 (DOCX 31 kb) The values of excess molar volumes, \( V_{\text{m}}^{\text{E}} \) (cm3·mol−1) and partial molar volumes (\( \bar{V}_{1} \),\( \bar{V}_{2} \)) for 2-methylpropan-2-ol (1) + ethane-1,2-diol at different temperatures are supplied in Table S1 and Table S2 respectively
Rights and permissions
About this article
Cite this article
Aniya, V., Kumari, A., Reddy, R. et al. Measurement and Modeling of Phase Equilibrium, Volumetric Properties and Molar Refractivity of 2-Methylpropan-2-ol + Ethane-1,2-diol. J Solution Chem 46, 1177–1201 (2017). https://doi.org/10.1007/s10953-017-0638-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-017-0638-3