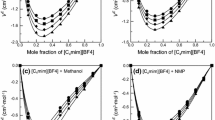
Abstract
Recently, ionic liquids have been combined with active pharmaceutical ingredients (APIs), and a third generation of ILs has emerged (API-ILs). The effect of a novel ionic liquid containing the ibuprofenate anion as an active pharmaceutical ingredient ionic liquid (API-IL) on the thermodynamic properties of two amino acids, glycine and l-alanine, have been studied. The densities, speeds of sound, viscosities and refractive indices of glycine and l-alanine in water and in aqueous solutions of an API-IL, 1-butyl-3-methylimidazolium ibuprofenate ([BMIM][Ibu]), have been determined at temperatures 288.15–318.15 K. The measured data have been used to calculate the standard partial molar volume \( V_{\phi }^{0} \), partial molar volume of transfer \( \Delta_{\text{tr}} V_{\phi }^{0} \), Hepler’s constant \( (\partial^{2} V_{\phi }^{0} /\partial T^{2} )_{p} \), apparent molar isentropic compressibility \( \kappa_{\phi } \), molar refraction R D, viscosity B coefficient (B) and hydration number parameters n H. All of these parameters are discussed in terms of the competitive interactions occurring between amino acids–[BMIM][Ibu] and amino acids–water.







Similar content being viewed by others
References
Geppi, M., Guccione, S., Mollica, G.: Molecular properties of ibuprofen and its solid dispersions with eudragit RL100 studied by solid-state nuclear magnetic resonance. Pharmaceut. Res. 22, 1544–1555 (2005)
Moniruzzama, M., Goto, M.: Application of ionic liquids: future solvents and reagents for pharmaceuticals. J. Chem. Eng. Jpn. 44, 370–381 (2013)
Bittner, B., Mountfieldm, R.J.: Formulations and relative activities for the oral administration of poorly water soluble in early drug delivery discovery animal studies. Pharm. Ind. 64, 800–807 (2002)
Bitter, B., Mountfield, R.: Intravenous administration of poorly soluble new drug entities in early drug discovery: the potential impact of formulation on pharmacokinetic parameters. J. Curr. Opinion Drug Discov. Develop. 5, 59–71 (2002)
Shamshina, J.L., Barber, P.S., Rogers, R.D.: Ionic liquids in drug delivery. Expert Opinion Drug Deliv. 10, 1367–1381 (2013)
Hough, W.L., Smiglak, M., Rodriguez, H., Swatloski, R.P., Spear, S.K., Daly, D.T., Pernak, J., Grisel, J.E., Carliss, R.D., Soutullo, M.D., Davis, J.H., Rogers, R.D.: The third evolution of ionic liquids: active pharmaceutical ingredients. New J. Chem. 31, 1429–1436 (2007)
Ferraz, R., Branco, L.C., Prudencio, C., Noronha, J.P., Petrovski, Z.: Ionic liquids as active pharmaceutical ingredients. Chem. Med. Chem. 6, 975–985 (2011)
Bica, K., Rijksen, C., Nieuwenhuyzen, M., Rogers, R.D.: In search of pure liquid salt forms of aspirin: ionic liquid approaches with acetylsalicylic acid and salicylic acid. Phys. Chem. Chem. Phys. 12, 2011–2017 (2010)
Stoimenovski, J., MacFarlane, D.R., Bica, K., Rogers, R.D.: Crystalline vs. ionic liquid salt forms of active pharmaceutical ingredients: a position paper. Pharmaceut. Res. 27, 521–526 (2010)
Viau, L., Tourne-Peteilh, C., Devoisselle, J., Vioux, A.: Ionogels as drug delivery system: one-step sol–gel synthesis using imidazolium ibuprofenate ionic liquid. Chem. Commun. 46, 228–230 (2010)
Fei, Z., Geldbach, T.J., Zhao, D., Dyson, P.J.: From dysfunction to bis-function: on the design and applications of functionalised ionic liquids. Chem. A Eur. J. 12(8), 2122–2130 (2006)
Nishi, N., Kawakami, T., Shigematsu, F., Yamamoto, M., Kakiuchi, T.: Fluorine-free and hydrophobic room-temperature ionic liquids, tetraalkylammonium bis(2-ethylhexyl) sulfosuccinates, and their ionic liquid–water two-phase properties. Green Chem. 8, 349–355 (2006)
Jencks, W.P.: Catalysis in Chemistry and Enzymology. McGraw Hill, New York (1969)
Von Hippel, P.H., Schleich, T.: Ion effects on the solution structure of biological macromolecules. Acc. Chem. Res. 2, 257–265 (1969)
Voet, D., Voet, J.G.: Biochemistry, 2nd edn. Wiley, New York (1995)
Hvidt, A., Westh, P.: Different views on the stability of protein conformations and hydrophobic effects. J. Solution Chem. 27, 395–402 (1998)
Lavrich, R.J., Torok, C.R., Tubergen, M.J.: Effect of the bulky side chain on the backbone structure of the amino acid derivative valinamide. J. Phys. Chem. A 106, 8013–8018 (2002)
Shekaari, H., Jebali, F.: Densities, viscosities, electrical conductances, and refractive indices of amino acid + ionic liquid ([BMIm]Br) + water mixtures at 298.15 K. J. Chem. Eng. Data 55, 2517–2523 (2010)
Shekaari, H., Jebali, F.: Densities and electrical conductances of amino acids + ionic liquid ([HMIm]Br) + H2O mixtures at 298.15 K. Fluid Phase Equilib. 295, 68–75 (2010)
Shekaari, H., Jebali, F.: Solute–solvent interactions of amino acids in aqueous 1-propyl-3-methylimidazolium bromide ionic liquid solutions at 298.15 K. J. Solution Chem. 39, 1409–1427 (2010)
Fang, S., Ren, D.H.: Effect of 1-ethyl-3-methylimidazolium bromide ionic liquid on the volumetric behavior of some aqueous l-amino acids solutions. J. Chem. Eng. Data 58, 845–850 (2013)
Roy, M.N., De, P., Sikdar, P.S.: Study of solvation consequences of α-amino acids in aqueous ionic liquid solution probed by physicochemical approach. Fluid Phase Equilib. 352, 7–13 (2013)
Vasantha, T., Kumar, A., Attri, P., Venkatesu, P., Devi, R.S.R.: Influence of biocompatible ammonium ionic liquids on the solubility of l-alanine and l-valine in water. Fluid Phase Equilib. 335, 39–45 (2012)
Vasantha, T., Kumar, A., Attri, P., Venkatesu, P., Devi, R.S.R.: The solubility and stability of amino acids in biocompatible ionic liquids. Lett. Protein Peptide Lett. 21, 15–24 (2014)
Singh, V., Chhotaray, P.K., Banipal, P.K., Banipal, T.S., Gardas, R.L.: Volumetric properties of amino acids in aqueous solutions of ammonium based protic ionic liquids. Fluid Phase Equilib. 385, 258–274 (2015)
Kumar, H., Singla, M., Jindal, R.: Interactions of glycine, l-alanine and l-valine with aqueous solutions of trisodium citrate at different temperatures: A volumetric and acoustic approach. J. Chem. Thermodyn. 67, 170–180 (2013)
Rima, F.R.: Monirul Islam, M., Nazrul Islam, M.: Excess volume of water in hydrate complexes of some α-amino acids. J. Chem. Eng. Data 58, 2991–2997 (2013)
Kumar, H., Kaur, K.: Effect of dipotassium hydrogen phosphate on thermodynamic properties of glycine and l-alanine in aqueous solutions at different temperatures. J. Chem. Thermodyn. 53, 86–92 (2012)
Sadeghi, R., Goodarzi, B.: Apparent molar volumes and isentropic compressibilities of transfer of l-alanine from water to aqueous potassium di-hydrogen citrate and tri-potassium citrate at T = (283.15 to 308.15) K. J. Mol. Liq. 14, 62–68 (2008)
Fortin, T.J., Laesecke, A., Freund, M., Outcalt, S.: Advanced calibration, adjustment, and operation of a density and sound speed analyzer. J. Chem. Thermodyn. 57, 276–285 (2013)
Holbrey, J.D., Seddon, K.R.: The phase behaviour of 1-alkyl-3-methylimidazolium tetrafluoroborates; ionic liquids and ionic liquid crystals. J. Chem. Soc. Dalton Trans. 13, 2133–2140 (1999)
Tourne-Peteilh, C., Devoisselle, J.M., Vioux, A., Judeinstein, P., Inc, M., Viau, L.: Surfactant properties of ionic liquids containing short alkyl chain imidazolium cations and ibuprofenate anions. Phys. Chem. Chem. Phys. 13, 15523–15529 (2011)
Hedwig, G.R., Reading, J.F., Lilley, T.H.: Aqueous solutions containing amino acids and peptides. Part 27. Partial molar heat capacities and partial molar volumes of some N-acetyl amino acid amides, some N-acetyl peptide amides and two peptides at 25 °C. J. Chem. Soc. Faraday Trans. 87, 1751–1758 (1991)
Kikuchi, K., Sakurai, M., Nitta, K.: Partial molar volumes and adiabatic compressibilities of amino acids in dilute aqueous solutions at 5, 15, 25, 35, and 45 °C. J. Chem. Eng. Data 40, 935–942 (1995)
Islam, M.N., Wadi, R.K.: Effects of the coating process on nanoscale Y2O3: Eu3+ powders. Phys. Chem. Liq. 41, 533–535 (2003)
Li, S.Z.F., Wang, B.H., Zhang, Y.M.: Partial molar volumes of some amino acids and a peptide in water, DMSO, NaCl, and DMSO/NaCl aqueous solutions. J. Chem. Thermodyn. 32, 805–819 (2000)
Banipal, T.S., Kaur, J., Banipal, P.K., Singh, K.: Study of interactions between amino acids and zinc chloride in aqueous solutions through volumetric measurements at T = (288.15 to 318.15) K. J. Chem. Eng. Data 53, 1803–1816 (2008)
Pal, A., Kumar, S.: Viscometric and volumetric studies of some amino acids in binary aqueous solutions of urea at various temperatures. J. Mol. Liq. 109, 23–31 (2004)
Kharakoz, D.P.: Volumetric properties of proteins and their analogs in diluted water solutions: 1. Partial volumes of amino acids at 15–55 °C. Biophys. Chem. 34, 115–125 (1989)
Hakin, A.W., Duke, M.M., Klassen, S.A., McKay, R.M., Preuss, K.E.: Apparent molar heat capacities and volumes of some aqueous solutions of aliphatic amino acids at 288.15, 298.15, 313.15, and 328.15 K. J. Chem. 72(2), 362–368 (1994)
Lippert, K., Galinski, E.A.: Enzyme stabilization be ectoine-type compatible solutes: protection against heating, freezing and drying. Appl. Microbiol Biotechnol. 37(1), 61–65 (1992)
Bhattacharya, M.M., Sengupta, M.: Ion-solvent interaction of amino acids: IV. Apparent molar volumes of amino acids in natural acidic and alkaline media at different temperatures. J. Indian Chem. Soc. 62, 959–964 (1985)
Millero, F.J.: Molal volumes of electrolytes. Chem. Rev. 71, 147–176 (1971)
Marcus, Y., Hefter, G.: Standard partial molar volumes of electrolytes and Ions in nonaqueous solvents. Chem. Rev. 104, 3405–3452 (2004)
Kumar, H., Singla, M., Jindal, R.: Solvation behavior of some amino acid compounds in aqueous solutions of trilithium citrate at different temperatures. J. Mol. Liq. 197, 301–314 (2014)
Dhondge, S.S., Zodape, S.P., Parwate, D.V.: Volumetric and viscometric studies of some drugs in aqueous solutions at different temperatures. J. Chem. Thermodyn. 48, 207–212 (2012)
Hepler, L.G.: Thermal expansion and structure in water and aqueous solutions. Can. J. Chem. 47, 4613–4617 (1969)
Banerjee, T., Kishore, N.: Interactions of some amino acids with aqueous tetraethylammonium bromide at 298.15 K: a volumetric approach. J. Solution Chem. 34, 137–153 (2005)
Bhat, R., Kishore, N., Ahluwalia, J.C.: Thermodynamic studies of transfer of some amino acids and peptides from water to aqueous glucose and sucrose solutions at 298.15 K. J. Chem. Soc. Faraday Trans. I(84), 2651–2655 (1988)
Wadi, R.K., Ramasami, P.: Partial molal volumes and adiabatic compressibilities of transfer of glycine and Dl-alanine from water to aqueous sodium sulfate at 288.15, 298.15 and 308.15 K. J. Chem. Soc. Faraday Trans. 93, 243–247 (1997)
Sadeghi, R., Goodarzi, B.: Apparent molar volumes and isentropic compressibilities of transfer of l-alanine from water to aqueous potassium di-hydrogen citrate and tri-potassium citrate at T = (283.15 to 308.15) K. J. Mol. Liq. 141, 62–68 (2008)
Gurney, R.W.: Ionic Process in Solution, Chap. 1. McGraw Hill, New York (1953)
Sharma, Kumar: S., Singh, G., Kumar, H., Kataria, R.: Study of solute–solute and solute–solvent interactions of N-acetyl glycine in aqueous d-fructose solutions at different temperatures. Thermochim. Acta 607, 1–8 (2015)
Majdan-Cegincara, R., Zafarani-Moattar, M.T., Shekaari, H.: The study of solute–solvent interactions in 1-butyl-1-methylpyrrolidinium trifluoromethanesulfonate + acetonitrile from solvent activity, density, speed of sound, viscosity, electrical conductivity and refractive index measurements. J. Mol. Liq. 203, 198–203 (2015)
Kumar, H., Kaur, K.: Volumetric, compressibility and UV spectral studies on interactions of amino acids with aqueous solutions of potassium dihydrogen phosphate at different temperatures. J. Solution Chem. 42, 592–614 (2013)
Sadeghi, R., Gholamireza, A.: Thermodynamics of the ternary systems: (water + glycine, l-alanine and l-serine + di-ammonium hydrogen citrate) from volumetric, compressibility, and (vapour + liquid) equilibria measurements. J. Chem. Thermodyn. 43, 200–215 (2011)
Sadheghi, R., Parhizkar, H.: Volumetric, isentropic compressibility and electrical conductivity of solutions of tri-sodium phosphate in 1-propanol + water mixed-solvent media over the temperature range of 283.15–303.15 K. Fluid Phase Equilib. 265, 173–183 (2008)
Lin, G.M., Bian, P.F., Lin, R.S.: The limiting partial molar volume and transfer partial molar volume of glycylglycine in aqueous sodium halide solutions at 298.15 K and 308.15 K. J. Chem. Thermodyn. 38, 144–151 (2006)
McMillan, W.G., Mayer, J.E.: The Statistical thermodynamics of multicomponent systems. J. Chem. Phys. 13, 276–305 (1945)
Krishnan, C.V., Friedman, H.L.: Enthalpies of alkyl sulfonates in water, heavy water, and water–alcohol mixtures and the interaction of water with methylene groups. J. Solution Chem. 2, 37–51 (1973)
Kumar, H., Singla, M., Jindal, R.: Viscometric measurements of dipeptides of alanine in aqueous solutions of antibacterial drug ampicillin at different temperatures. J. Mol. Liq. 191, 183–188 (2014)
Kumar, H., Singla, M., Jindal, R.: Interactions of amino acids in aqueous triammonium citrate solutions at different temperatures: A viscometric approach. J. Mol. Liq. 199, 385–392 (2014)
Jindal, R., Singla, M., Kumar, H.: Transport behavior of aliphatic amino acids glycine/l-alanine/l-valine and hydroxyl amino acids l-serine/l-threonine in aqueous trilithium citrate solutions at different temperatures. J. Mol. Liq. 206, 343–349 (2015)
Jones, G., Dole, M.: The viscosity of aqueous solutions of strong electrolytes with special reference to barium chloride. J. Am. Chem. Soc. 51, 2950–2964 (1929)
Zhao, Q., Sun, Z.J., Zhang, Q., Xing, S.K., Liu, M., Sun, D.Z.: Densities and apparent molar volumes of myoinositol in aqueous solutions of alkaline earth metal salts at different temperatures. Thermochim. Acta 487, 1–7 (2009)
Sarma, T.S., Ahiuwalia, J.C.: Experimental studies on the structures of aqueous solutions of hydrophobic solutes. Chem. Soc. Rev. 2, 203–232 (1973)
Yan, Z., Wang, J., Liu, W., Lu, J.: Apparent molar volumes and viscosity B-coefficients of some α-amino acids in aqueous solutions from 278.15 to 308.15 K. Thermochim. Acta 334, 17–27 (1999)
Wadi, R.K., Goyal, R.K.: Temperature dependence of apparent molar volumes and viscosity B-coefficients of amino acids in aqueous potassium thiocyanate solutions from 15 to 35 °C. J. Solution Chem. 21, 163–170 (1992)
Banipal, T.S., Kaur, N., Kaur, A., Gupta, M., Banipal, P.K.: Effect of food preservatives on the hydration properties and taste behavior of amino acids. Food Chem. 181, 339–346 (2015)
Feakins, D., Waghorne, W.E., Lawrence, K.G.: The viscosity and structure of solutions. Part 1. A new theory of the Jones–Dole B-coefficient and the related activation parameters: application to aqueous solutions. J. Chem. Soc. Faraday Trans. I(82), 563–568 (1986)
Tjahjono, M., Garland, M.: On the Determination of partial molar volumes, partial molar refractions, mean electronic polarizabilities and effective molecular radii from dilute multi-component data alone using response surface models. J. Solution Chem. 36, 221–236 (2007)
Robinson, R.H., Stokes, R.H.: Electrolyte Solutions. Butterworth, London (1955)
Acknowledgments
The authors wish to thank the graduate council of the University of Tabriz for financial support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shekaari, H., Zafarani-Moattar, M.T. & Mirheydari, S.N. Effect of 1-Butyl-3-methylimidazolium Ibuprofenate as an Active Pharmaceutical Ingredient Ionic Liquid (API-IL) on the Thermodynamic Properties of Glycine and l-Alanine in Aqueous Solutions at Different Temperatures. J Solution Chem 45, 624–663 (2016). https://doi.org/10.1007/s10953-016-0462-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-016-0462-1