Abstract
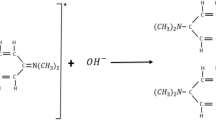
In order to study the chemical oscillatory behavior and mechanism of a new chlorine dioxide–iodine–acetylacetone reaction system, a series of experiments were carried out by using UV–Vis and online FTIR spectrophotometric methods. The initial concentrations of acetylacetone, chlorine dioxide, iodine, sulfuric acid, and the pH value have considerable influence on the oscillations observed at wavelength of 350 nm for the starch–triiodide ion complex (\({\text{QI}{_{3}^-}}\)). There is a pre-oscillatory or induction stage, the amplitude and the number of oscillations are associated with the initial concentration of reactants. Equations were obtained for the starch–triiodide ion complex (\({\text{QI}{_{3}^-}}\)) reaction rate change with reaction time and the initial concentrations in the oscillation stage. The oscillation reaction can be accelerated by increasing the reaction temperature. The apparent activation energies at the induction stage and the oscillation stage are 45.64 and 12.39 kJ·mol−1, respectively. The intermediates were detected by the online FTIR analysis. Based upon the experimental data in this work and in the literature, a plausible reaction mechanism was proposed for the oscillation reaction.





Similar content being viewed by others
References
Dolnik, M., Epstein, I.R.: A coupled chemical burster: the chlorine dioxide–iodide reaction in two flow reactors. J. Chem. Phys. 98, 1149–1155 (1993)
De Kepper, P., Epstein, I.R., Kustin, K., Orbán, M.: Batch oscillations and spatial wave patterns in chlorite oscillating systems. J. Phys. Chem. 86, 170–171 (1982)
De Kepper, P., Epstein, I.R.: A mechanistic study of oscillations and bistability in the Briggs–Rauscher reaction. J. Am. Chem. Soc. 104, 49–55 (1982)
De Kepper, P., Boissonade, J., Epstein, I.R.: Chlorite–iodide reaction: a versatile system for the study of nonlinear dynamical behavior. J. Phys. Chem. 94, 6525–6536 (1990)
Lengyel, I., Rábai, G., Epstein, I.R.: Batch oscillation in the reaction of chlorine dioxide with iodine and malonic acid. J. Am. Chem. Soc. 112, 4606–4607 (1990)
Lengyel, I., Rábai, G., Epstein, I.R.: Experimental and modeling study of oscillations in the chlorine dioxide–iodine–malonic acid reaction. J. Am. Chem. Soc. 112, 9104–9110 (1990)
Lengyel, I., Li, J., Kustin, K., Epstein, I.R.: Rate constants for reactions between iodine- and chlorine-containing species: a detailed mechanism of the chlorine dioxide/chlorite–iodide reaction. J. Am. Chem. Soc. 118, 3708–3719 (1996)
Lengyel, I., Epstein, I.R.: Modeling of turing structures in the chlorite–iodide–malonic acid–starch reaction system. Science 251(4994), 650–652 (1991)
Lengyel, I., Kadar, S., Epstein, I.R.: Transient turing structures in a gradient-free closed system. Science 259(5094), 493–495 (1993)
Fabian, I., Gordon, G.: The kinetics and mechanism of the chlorine dioxide–iodide ion reaction. Inorg. Chem. 36, 2494–2497 (1997)
Szalai, I., De Kepper, P.: Turing patterns, spatial bistability, and front instabilities in a reaction–diffusion system. J. Phys. Chem. A 108, 5315–5321 (2004)
Strier, D.E., De Kepper, P., Boissonade, J.: Turing patterns, spatial bistability, and front interactions in the [ClO2, I2, I−, CH2(COOH)2] reaction. J. Phys. Chem. A 109, 1357–1363 (2005)
Riaz, S.S., Ray, D.S.: Spiral pattern in chlorite–iodide–malonic acid reaction: a theoretical and numerical study. J. Phys. Chem. 123, 174506.1–174506.5 (2005)
Long, D.A., Chodroff, L., O’Neal, T.M., Hemkin, S.: A true chemical clock: serially coupled chlorite–iodide oscillators. Chem. Phys. Lett. 447, 340–344 (2007)
Shi, L., Li, W., Wang, F.: Experimental study of a closed system in the chlorine dioxide–iodine–malonic acid–sulfuric acid oscillation reaction by UV–Vis spectrophotometric method. J. Solution Chem. 38, 571–588 (2009)
Gao, J., Shi, L., Wei, Q., Li, X., Li, Z.: Chlorine dioxide–iodine–ethyl 2-chloroacetoacetate oscillation reaction investigated by UV–Vis and online FTIR spectrophotometric methods. J. Solution Chem. 43, 1078–1092 (2014)
Shi, L., Qian, Y., Lin, S., Yang, B., Li, N., Liu, J.: Sodium chlorite–iodine–methyl acetoacetate oscillatory reaction investigated by UV–Vis spectrophotometric method. J. Iran. Chem. Soc. 10, 21–28 (2013)
Shi, L., Li, N., Liu, J., Qian, Y., Yang, B., Lin, S.: Sodium chlorite–iodine–methyl acetoacetate oscillatory reaction investigated by UV–Vis spectrophotometry. Indian J. Chem. Sect. A 52, 226–231 (2013)
Powling, J., Bernstein, H.J.: The effect of solvents on tautomeric equilibria. J. Am. Chem. Soc. 73, 4353–4356 (1951)
Wyman, D.P., Kaufman, P.R., Freeman, W.R.: The chlorination of active hydrogen compounds with sulfuryl chloride. II. Esters, nitriles, nitro compounds, and aldehydes. J. Org. Chem. 29, 2706–2710 (1964)
Cieciuch, R.F.W., Westheimer, F.W.: Halide catalysis in the bromination of deoxybenzoin. J. Am. Chem. Soc. 85, 2591–2595 (1963)
Chen, E.Y., Song, X.H., Zhang, X.H.: A Study on isomerization of acetylacetone’s two isomers. J. Qiqihar Teach. Coll. (Nat. Sci.) 15, 31–32 (1995)
Acknowledgments
The authors thank the Shandong Provincial Natural Science Foundation (No. ZR2009BM007), and the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry, China, for financial support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, D., Shi, L., Chen, J. et al. Chlorine Dioxide–Iodine–Acetylacetone Oscillation Reaction Investigated by UV–Vis and Online FTIR Spectrophotometric Methods. J Solution Chem 45, 81–94 (2016). https://doi.org/10.1007/s10953-016-0433-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-016-0433-6