Abstract
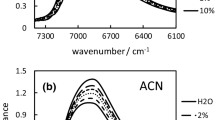
In order to investigate the effect of dissolution of salts on the hydrogen-bonded network in liquid water, near-infrared absorption spectra of aqueous solutions of 16 salts, containing Na+ as common cation, were measured in the region where the first overtone of the –OH stretching mode of water is observed. Although the spectral variations of water resulting from dissolution of a salt is dependent on the kind of salt, principal component analysis of the observed spectra revealed that all spectral variations for the 16 salts were almost reproducible with only three components. The first component corresponds to the average of the observed spectra, while the other two components are responsible for the variations. The second component, which almost coincides with the component of the spectral variation of water from changes in temperature, was found to explain mainly the spectral changes by salts that destroy the hydrogen-bonded network. On the other hand, the third component, which includes the spectral changes at a lower wavenumber region than the second component, was found to mainly explain the spectral variation from the salts that expand the hydrogen-bonded network. These results suggest that observed spectral variations are not due to direct interaction between ions and water molecules, but due to the change of the hydrogen-bonded network because all variations produced by these 16 salts can be explained by only two components. The results suggest also that the mechanisms of destruction and expansion of the hydrogen-bonded network by the anions may be different.







Similar content being viewed by others
References
Murrell, J.N., Jenkins, A.D.: Properties of Liquids and Solutions, 2nd edn. Wiley, Chichester (1994)
Marcus, Y.: Effect of ions on the structure of water. Pure Appl. Chem. 82, 1889–1899 (2010)
Czarnik-Matusewicz, B., Pilorz, S.: Study of the temperature-dependent near-infrared spectra of water by two-dimensional correlation spectroscopy and principal components analysis. Vib. Spectrosc. 40, 235–245 (2006)
Gowen, A.A., Amigo, J.M., Tsenkova, R.: Characterization of hydrogen bond perturbations in aqueous systems using aquaphotomics and multivariate curve resolution-alternating least squares. Anal. Chim. Acta 759, 8–20 (2013)
Lin, J., Brown, C.W.: Novel applications of near-infrared spectroscopy of water and aqueous solutions from physical chemistry to analytical chemistry. Trends Anal. Chem. 13, 320–326 (1994)
Lin, J., Zhou, J., Brown, C.W.: Identification of electrolytes in aqueous solutions from near-IR spectra. Appl. Spectrosc. 50, 444–448 (1996)
Pegau, W.S., Gray, D., Zaneveld, J.R.V.: Absorption and attenuation of visible and near-infrared light in water: dependence on temperature and salinity. Appl. Opt. 36, 6035–6046 (1997)
Frost, V.J., Molt, K.: Analysis of aqueous solutions by near-infrared spectrometry (NIRS) III. Binary mixtures of inorganic salts in water. J. Mol. Struct. 410–411, 573–579 (1997)
Saitow, K., Kobayashi, K., Nishikawa, K.: How are hydrogen bonds perturbed in aqueous NaClO4 solutions depending on the concentration?: A near infrared study of water. J. Solution Chem. 33, 689–698 (2004)
Omar, A.F., Atan, H., Matjafri, M.Z.: NIR spectroscopic properties of aqueous acids solutions. Molecules 17, 7440–7450 (2012)
Sebe, F., Nishikawa, K., Koga, Y.: Spectrum of excess partial molar absorptivity. Part II: A near infrared spectroscopic study of aqueous Na-halides. Phys. Chem. Chem. Phys. 14, 4433–4439 (2012)
Chang, K., Jung, Y.M., Chung, H.: Two-dimensional correlation analysis to study variation of near-infrared water absorption bands in the presence of inorganic acids. J. Mol. Struct. 1069, 122–126 (2014)
Davidian, A.G., Kudrev, A.G., Myund, L.A., Khripun, M.K.: Near infrared spectral studies of aqueous solutions of metal perchlorates in groups IA, IIA, IIB, IIIA and IIIB of the periodic table. J. Near Infrared Spectrosc. 22, 27–34 (2014)
Sobinaa, E.P., Neudachinaa, L.K., Medvedevskikh, S.V., Medvedevskikh, MYu.: Effect of the nature of ions on the position of the absorption bands of water OH bonds in diffuse-reflection spectra in the near-infrared region. Russ. J. Phys. Chem. A 85, 1168–1173 (2011)
Davidian, A.G., Kudrev, A.G., Myund, L.A., Khlynova, O.S., Khripun, M.K.: Structure of aqueous electrolyte solutions estimated by near infrared spectroscopy and chemometric analysis of spectral data. Russ. J. Gen. Chem. 84, 1877–1887 (2014)
Gowena, A.A., Marini, F., Tsuchisaka, Y., DeLuca, S., Bevilacqua, M., O’Donnell, C., Downey, G., Tsenkova, R.: On the feasibility of near infrared spectroscopy to detect contaminants in water using single salt solutions as model systems. Talanta 31, 609–618 (2015)
Heiman, A., Licht, S.: Fundamental baseline variations in aqueous near-infrared analysis. Anal. Chim. Acta 394, 135–147 (1999)
Hasegawa, T.: Chemometrics in Infrared Spectroscopic Analysis. In: Tasumi, M. (ed.) Introduction to Experimental Infrared Spectroscopy, pp. 97–113. Wiley, Chichester (2015)
Ohtaki, H., Radnai, T.: Structure and dynamics of hydrated ions. Chem. Rev. 93, 1157–1204 (1993)
Bakker, H.J.: Structural dynamics of aqueous salt solutions. Chem. Rev. 108, 1456–1473 (2008)
Marcus, Y.: Effect of ions on the structure of water: structure making and breaking. Chem. Rev. 109, 1346–1370 (2009)
Zhang, Y., Cremer, P.S.: Interactions between macromolecules and ions: the Hofmeister series. Curr. Opin. Chem. Biol. 10, 658–666 (2006)
Kunz, W.: Specific ion effects in colloidal and biological systems. Curr. Opin. Colloid Interface Sci. 15, 34–39 (2010)
Omta, A.W., Kropman, M.F., Woutersen, S., Bakker, H.J.: Negligible effect of ions on the hydrogen-bond structure in liquid water. Science 301, 347–349 (2003)
Smith, J.D., Saykally, R.J., Geissler, P.L.: The effects of dissolved halide anions on hydrogen bonding in liquid water. J. Am. Chem. Soc. 129, 13847–13856 (2007)
Bruni, F., Imberti, S., Mancinelli, R., Ricci, M.A.: Aqueous solutions of divalent chlorides: ions hydration shell and water structure. J. Chem. Phys. 136, 064520 (2012)
Mancinelli, R., Botti, A., Bruni, F., Ricci, M.A., Soper, A.K.: Hydration of sodium, potassium, and chloride ions in solution and the concept of structure maker/breaker. J. Phys. Chem. B 111, 13570–13577 (2007)
Kondoh, M., Ohshima, Y., Tsubouchi, M.: Ion effects on the structure of water studied by terahertz time-domain spectroscopy. Chem. Phys. Lett. 591, 317–322 (2014)
Waluyo, I., Huang, C., Nordlund, D., Weiss, T.M., Pettersson, L.G.M., Nilsson, A.: Increased fraction of low-density structures in aqueous solutions of fluoride. J. Chem. Phys. 134, 224507 (2011)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Uchida, N., Yoshimura, N. & Takayanagi, M. Variation of the Near-Infrared Spectrum of Water from Dissolved Salts. J Solution Chem 44, 2167–2178 (2015). https://doi.org/10.1007/s10953-015-0399-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-015-0399-9