Abstract
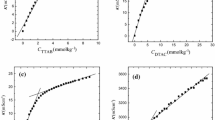
Synergistic interactions between cetrimide and sodium dodecyl sulfate (SDS) were studied at three different temperatures. Aqueous solutions of pure cetrimide, SDS and their mixtures were investigated conductometrically. The mixed surfactants exhibited nonideal behavior as the CMCs are less than the ideal CMC values calculated by using Clint’s equation. In addition, Rubingh’s model was used and various parameters such as the activity coefficients f 1 and f 2, micelllar mole fraction, X, interaction parameter, β, excess Gibbs energy and enthalpy of mixing, \( \Delta G_{\text{ex}} \) and \( \Delta H_{\text{ex}} \), respectively, were calculated. The values of f 1, f 2, and β indicate strong interaction between cetrimide and SDS and the interaction increases with increasing mole fraction of SDS in the mixture. Negative β values and |β| > |ln(CMC 1 /CMC 2)| clearly confirm synergism in the surfactant mixture. Negative \( \Delta G_{\text{ex}}^{{}} \) and \( \Delta H_{\text{ex}}^{{}} \) values of the mixed systems at different bulk mole fractions also reflect the behaviors of β and f. The values of the standard Gibbs energy and enthalpy of mixed micelle formation are negative, suggesting that micellization is thermodynamically favorable and exothermic in nature. Larger positive contributions of the entropic component, \( T\Delta S_{\text{m}}^{ 0} \), than the enthalpic component, \( \Delta H_{\text{m}}^{ 0} \), to \( \Delta G_{\text{m}}^{ 0} \) are ascribed to the destruction of the structured water surrounding the hydrophobic groups of the surfactants when these groups are transferred from the bulk into the interior of the micelle.





Similar content being viewed by others
References
Sohn, B.H., Choi, J.M., Yoo, S.I., Yun, S.H., Zin, W.C., Jung, J.C., Kanehara, M., Hirata, T., Teranishi, T.: Directed self-assembly of two kinds of nanoparticles utilizing monolayer films of diblock copolymer micelles. J. Am. Chem. Soc. 125, 6368–6369 (2003)
Jaramillo, T.F., Baeck, S.H., Cuenya, B.R., McFarland, E.W.: Catalytic activity of supported Au nanoparticles deposited from block copolymer micelles. J. Am. Chem. Soc. 125, 7148–7149 (2003)
Khan, A.B., Ali, M., Malik, N.A., Ali, A., Patel, R.: Role of 1-methyl-3-octylimidazolium chloride in the micellization behavior of amphiphilic drug amitriptyline hydrochloride. Colloids Surf. B 112, 460–465 (2013)
Zhang, H., Annunziata, O.: Diffusion of an ionic drug in micellar aqueous solution. Langmuir 25, 3425–3434 (2009)
Tiwary, L.K., Mandal, A., Alam, M.S., Thennarasu, S., Mandal, A.B.: Thermodynamics studies on tyrosine–hydantoin drug–cetyltrimethylammonium bromide mixed micellar system. Colloids Surf. B 82, 126–133 (2011)
Alexandridis, P., Lindman, B.: Amphiphilic Block Copolymers: Self-assembly and Applications. Elsevier, Amsterdam (2000)
Li, C., Wallace, S.: Polymer-drug conjugates: recent development in clinical oncology. Adv. Drug Delivery Rev. 60, 886–898 (2008)
Rosen, M.J.: Surfactants and Interfacial Phenomena, 3rd edn. Wiley-Interscience, New York (2004)
Joshi, T., Mata, J., Bahadur, P.: Micellization and interaction of anionic and nonionic mixed surfactant systems in water. Colloids Surf. A 260, 209–215 (2005)
Hao, L.S., Deng, Y.T., Zhou, L.S., Ye, H., Nan, Y.Q., Hu, P.: Mixed micellization and the dissociated Margules model for cationic/anionic surfactant systems. J. Phys. Chem. B. 116, 5213–5225 (2012)
Ghosh, S., Burman, A.D., De, G.C., Das, A.R.: Interfacial and self-aggregation of binary mixtures of anionic and nonionic amphiphiles in aqueous medium. J. Phys. Chem. B. 115, 11098–11112 (2011)
Haque, M.E., Das, A.R., Rakshit, A.K., Moulik, S.P.: Properties of mixed micelles of binary surfactant combinations. Langmuir 12, 4084–4089 (1996)
Zhou, Q., Rosen, M.J.: Molecular interactions of surfactants in mixed monolayers at the air/aqueous solution interface and in mixed micelles in aqueous media: the regular solution approach. Langmuir 19, 4555–4562 (2003)
Singh, O.G., Ismail, K.: Micellization behavior of mixtures of sodium dioctylsulfosuccinate with sodium dodecylsulfate in water. J. Surfact. Deterg. 11, 89–96 (2008)
Moore, S.E., Mohareb, M., Moore, S.A., Palepu, R.M.: Conductometric and fluorometric investigations on the mixed micellar systems of cationic surfactants in aqueous media. J. Colloid Interface Sci. 304, 491–496 (2006)
Holland, P., Rubingh, D.N.: Nonideal multicomponent mixed micelle model. J. Phys. Chem. 87, 1984–1990 (1983)
Moallemi, M., Sohrabi, B., Fazehi, S.: Electrolyte effect on adsorption and the phase transition from microstructures to nanostructures in ionic/ionic surfactants mixture. J. Colloid Interface Sci. 361, 159–169 (2011)
Sohrabi, B., Gharibi, H., Tajik, B., Javadian, S., Hashemianzadeh, M.: Molecular interactions of cationic and anionic surfactants in mixed monolayers and aggregates. J. Phys. Chem. B 112, 14869–14876 (2008)
Bergstrom, M.: Synergistic effects in mixtures of an anionic and a cationic surfactant. Langmuir 17, 993–998 (2001)
Bergstrom, M., Eriksson, J.C.: A theoretical analysis of synergistic effects in mixed surfactant systems. Langmuir 16, 7173–7181 (2000)
Kabir-ud-Din, Rub, M.A., Naqvi, A.Z.: Mixed micelle formation between amphiphilic drug amitriptyline hydrochloride and surfactants (conventional and gemini) at 293.15 − 308.15 K. J. Phys. Chem. B 114, 6354–6364 (2010)
Barry, B.W., Morrison, J.C., Russel, G.F.J.: Prediction of the critical micelle concentration of mixtures of alkyltrimethylammonium salts. J. Colloid Interface Sci. 33, 554–561 (1970)
Attwood, D., Patel, H.K.: Composition of mixed micellar systems of cetrimide and chlorhexidine digluconate. Int. J. Pharmaceut. 49, 129–134 (1989)
Barry, B.W., Russel, G.F.J.: Prediction of micellar molecular weights and thermodynamics of micellization of mixtures of alkyltrimethylammonium salts. J. Colloid Interface Sci. 40, 174–194 (1972)
The European Agency for the Evaluation of Medicinal Products: Committee for Veterinary Medicinal Products. 7 Westferry Circus, Canary Wharf, London E14 4HB, UK
Mayers, D.: Surfactant Science and Technology, 3rd edn. Wiley-Interscience, Whiley, New Jersey (2006)
Ali, A., Nabi, F., Malik, N.A., Tasneem, S., Uzair, S.: Study of micellization of sodium dodecyl sulfate in non-aqueous media containing lauric acid and dimethylsulfoxide. J. Surfact. Deterg. 17, 151–160 (2014)
Clint, J.H.: Micellization of mixed nonionic surface active agents. J. Chem. Soc. Faraday Trans. 71, 1327–1334 (1975)
Holland, P.M.: Mixed surfactant systems. In: Holland, P.M., Rubingh, D.N. (eds.) ACS Symposium Series 501. American Chemical Society, Washington, DC (1992)
Rosen, M.J., Hua, X.Y.: Surface concentrations and molecular interactions in binary mixtures of surfactants. J. Colloid Interface Sci. 86, 164–172 (1982)
Lide, J.E., Zwolenik, J.S., Fuoss, R.M.: Calibration of conductance cells at 25° with aqueous solutions of potassium chloride. J. Am. Chem. Soc. 81, 1557–1559 (1959)
Lucassen-Reynders, E.H.: Anionic Surfactants-Physical Chemistry of Surfactant Action. Marcel Dekker, New York (1981)
Benerrou, M., Bales, B.L., Zana, R.: Effect of the nature of the counterion on the properties of anionic surfactants. 1. Cmc, ionization degree at the cmc and aggregation number of micelles of sodium, cesium, tetramethylammonium, tetraethylammonium, tetrapropylammonium, and tetrabutylammonium dodecyl sulfates. J. Phys. Chem. B 107, 13432–13440 (2003)
Ali, A., Malik, N.A., Uzair, S., Ali, M.: Conductometric and fluorometric studies of sodium dodecyl sulfate in aqueous solution and in the presence of amino acids. Mol. Phys. 112, 2681–2693 (2014)
Motomura, K., Yamanaka, M., Aratono, M.: Thermodynamic consideration of the mixed micelle of surfactants. Colloid Polym. Sci. 262, 948–955 (1984)
Fang, L., Gan-Zuoi, L., Jian-Bo, C.: Synergism in mixed zwitterionic–anionic surfactant solutions and the aggregation numbers of the mixed micelles. Colloids Surf. A 145, 167–174 (1998)
Nabel, A.N., Ahmad, M.E.S.: Interaction between cationic and conventional nonionic surfactants in the mixed micelle and monolayer formed in aqueous medium. Quim. Nova 34, 1007–1013 (2011)
Gil, H.N., Lee, B.H.: Study on the micellization of DPC/Brij 35 mixed surfactant systems by the conductivity method. J. Korean Chem. Soc. 52, 461–467 (2008)
Tadros, T.F.: Applied Surfactants-Principles and Applications. Wiley-VCH, Germany (2005)
Nightangle Jr, E.R.: Phenomenological theory of ion solvation. Effective radii of hydrated ions. J. Phys. Chem. 63, 1381–1387 (1959)
Mehta, S.K., Bhasin, K.K., Chauhan, R., Dham, S.: Effect of temperature on critical micelle concentration and thermodynamic behavior of dodecyldimethylethylammonium bromide and dodecyltrimethylammonium chloride in aqueous media. Colloids Surf. A 255, 153–157 (2005)
Acknowledgments
Nisar Ahmad Malik and Sahar Uzair are thankful to UGC (University Grants Commission), Government of India, for providing scholarships in the form of BSR (Basic Scientific Research).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ali, A., Malik, N.A., Uzair, S. et al. Conductometric Study of the Interaction of Cetrimide with Sodium Dodecyl Sulfate in Aqueous Medium. J Solution Chem 44, 1640–1654 (2015). https://doi.org/10.1007/s10953-015-0370-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-015-0370-9