Abstract
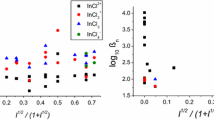
A study of the association between copper(II) and sulfate ions in aqueous solution has been made using copper ion-selective electrode potentiometry at constant ionic strengths (I) of 0.05, 0.1, 0.25, 0.5, 1.0, 3.0 and 5.0 mol·L−1 in NaClO4 media at 25 °C. Only one complex was detected, corresponding to the equilibrium: \( {\text{Cu}}^{ 2+ } ({\text{aq}}) + {\text{SO}}_{4}^{2 - } ({\text{aq}}) \rightleftarrows {\text{CuSO}}_{4}^{0} ({\text{aq}}). \) No higher order complexes were detected even at sulfate/copper(II) concentration ratios of up to 1,000. The present potentiometric values of log10 K 1(I) are shown to be consistently higher than those obtained by UV–Vis spectrophotometry because of the failure of the latter technique to detect all of the solvent-separated ion pairs present. Extrapolation of log10 K 1(I) to infinite dilution using an extended Guggenheim equation yielded a standard state value of log10 \( K_{1} \{ {\text{CuSO}}_{4}^{0} ({\text{aq}})\} = 2.32 \pm 0.09 \), which is in excellent agreement with a recent IUPAC-recommended value.


Similar content being viewed by others
References
Hayes, W.J.: Pesticides Studied in Man. William & Wilkins, Baltimore (1982)
Worthing, C.R. (ed.): The Pesticide Manual. British Crop Protection Council, Croydon (1983)
Hartley, D., Kidd, H. (eds.): The Agrochemicals Handbook. Royal Society of Chemistry, Nottingham (1983)
Nriagu, J.O. (ed.): Copper in the Environment. Wiley, New York (1979)
Brennan, R.F.: Effectiveness of some copper compounds applied as foliar sprays in alleviating copper deficiency of wheat grown on copper-deficient soils of Western Australia. Aust. J. Exp. Agric. 30, 687–691 (1990)
Kroschwitz, J. (ed.): Kirk-Othmer Encyclopaedia of Chemical Technology, 5th edn. Wiley, Hoboken (2004)
Biswas, A.K., Davenport, W.G.: Extractive Metallurgy of Copper, 3rd edn. Pergamon/Elsevier, Oxford (1994)
Pillay, B., Newman, J.: Modeling diffusion and migration in dilute electrochemical systems using the quasi-potential transformation. J. Electrochem. Soc. 140, 414–420 (1993)
Volgin, V.M., Davydov, A.D.: The limiting current density of copper electrodeposition on vertical electrode under the conditions of electrolyte natural convection. Elektrokhimiya 44, 496–507 (E: 459–469) (2008)
Powell, K.J., Brown, P.L., Byrne, R.H., Gajda, T., Hefter, G., Sjoberg, S., Wanner, H.: Chemical speciation of environmentally significant metals with inorganic ligands. Part 2. The Cu2+–OH−, Cl−, CO3 2−, SO4 2−, and PO4 3− systems. Pure Appl. Chem. 79, 895–950 (2007)
Hefter, G.: When spectroscopy fails: the measurement of ion pairing. Pure Appl. Chem. 78, 1571–1586 (2006)
Jeffery, H., Bassett, L., Mendham, J., Denney, R.C.: Vogel’s Textbook of Quantitative Chemical Analysis, 5th edn. Longman, New York (1989)
Anonymous: Ion-Selective Electrodes Manual. Metrohm Ltd., Heraus (2006)
May, P.M., Murray, K., Williams, D.R.: The use of glass electrodes for the determination of formation constants. II. Simulation of titration data. Talanta 32, 483–489 (1985)
May, P.M., Murray, K., Williams, D.R.: The use of glass electrodes for the determination of formation constants—III. Optimization of titration data: the ESTA library of computer programs. Talanta 35, 825–830 (1988)
Hefter, G.T.: Use of lithium perchlorate media in the study of protolytic equilibria. J. Solution Chem. 13, 179–190 (1984)
Buchner, R., Capewell, S.G., Hefter, G., May, P.M.: Ion-pair and solvent relaxation processes in aqueous Na2SO4 solutions. J. Phys. Chem. B 103, 1185–1192 (1999)
Capewell, S.G., Hefter, G.T., May, P.M.: Association constants for the NaSO4 − ion pair in concentrated cesium chloride solutions. Talanta 49, 25–30 (1999)
Rudolph, W.W., Irmer, G., Hefter, G.T.: Raman spectroscopic investigation of speciation in MgSO4(aq). Phys. Chem. Chem. Phys. 5, 5253–5261 (2003)
Buchner, R., Chen, T., Hefter, G.: Complexity in “simple” electrolyte solutions: ion pairing in MgSO4(aq). J. Phys. Chem. B 108, 2365–2375 (2004)
Powell, K.J., Brown, P.L., Byrne, R.H., Gajda, T., Hefter, G., Leuz, A.-K., Sjoberg, S., Wanner, H.: Chemical speciation of environmentally significant metals with inorganic ligands. Part 4: The Cd2+–OH−, Cl−, CO3 2−, SO4 2−, and PO4 3− systems. Pure Appl. Chem. 83, 1163–1214 (2011)
Powell, K.J., Brown, P.L., Byrne, R.H., Gajda, T., Hefter, G., Leuz, A.-K., Sjoberg, S., Wanner, H.: Chemical speciation of environmentally significant metals with inorganic ligands. Part 3. The Pb2+–OH−, Cl−, CO3 2−, SO4 2−, and PO4 3− systems. Pure Appl. Chem. 81, 2425–2476 (2009)
Powell, K.J., Brown, P.L., Byrne, R.H., Gajda, T., Hefter, G., Leuz, A.-K., Sjoberg, S., Wanner, H.: Chemical speciation of environmentally significant metals with inorganic ligands. Part 5: The Zn2+ + OH–, Cl–, CO3 2−, SO4 2−, and PO4 3− systems. Pure Appl. Chem. 85, 2249–2311 (2013)
Fronaeus, S.: Komplexsystem Hos Kopper, p. 139, Gleerupska Univ. Bokhandeln, Lund (1948); cited in Sillen, L.G., Martell, A.E. (eds.) Stability Constants of Metal-Ion Complexes. Special Publication No. 17. Chemical Society, London (1964)
Näsänen, R., Lumme, P.: Potentiometric studies on the equilibria of some copper(II)-hydroxysalts in aqueous salt solutions, and involved complex formation. Acta Chem. Scand. 5, 13–22 (1951)
Kenttämaa, J.: A cryoscopic method of studying the incomplete dissociation of strong electrolytes in aqueous solutions. Suomen Kemi. B 29, 59–64 (1956)
Akilan, C.: Thermodynamic and related studies of aqueous copper(II) sulfate solutions. Ph. D. Thesis, Murdoch University, Australia (2008)
Acknowledgments
The authors thank Andreas Nazet, University of Regensburg, Germany, for graphics assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Akilan, C., May, P.M. & Hefter, G. A Potentiometric Study of the Association of Copper(II) and Sulfate Ions in Aqueous Solution at 25 °C. J Solution Chem 43, 885–892 (2014). https://doi.org/10.1007/s10953-014-0170-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-014-0170-7