Abstract
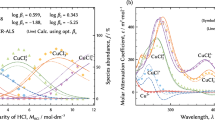
Knowledge of the thermodynamic properties of aqueous copper(II) chloride complexes is important for understanding and quantitatively modeling trace copper behavior in hydrometallurgical extraction processing. In this paper, UV–Vis spectra data of Cu(II) chloride solutions with various salinities (NaCl, 0–5.57 mol·kg−1) are collected at 25 °C. The concentration distribution of Cu–Cl species is in good agreement with those calculated by a reaction model (RM). The simple hydrated ion, Cu2+, is dominant at low concentration, whereas [CuCl]+, [CuCl2]0 and [CuCl3]− become increasingly important as the chloride concentration rises. Moreover, the RM calculation suggests the present of a small amount of [CuCl4]2−. The de-convoluted molar spectrum of each species is in excellent agreement with our previous theoretical results predicted by time-dependent density functional theory treatment of aqueous Cu-containing systems. The formation constants for these copper chloride complexes have been reported and are to be preferred, except log10 K 2 ([CuCl2]0).






Similar content being viewed by others

References
Brugger, J., McPhail, D.C., Black, J., Spiccia, L.: Complexation of metal ions in brines: application of electronic spectroscopy in the study of the Cu(II)–LiCl–H2O system between 25 and 90 °C. Geochim. Cosmochim. Acta 65, 2691–2708 (2001)
Gunton, C.: The role of salinity on the formation of geochemical anomalies in the regolith. In: Roach, I.C. (ed.) Advances in Regolith: Proceedings of the CRC LEME Regional Regolith Symposia, pp. 154–158, CRC LEME, West Australia (2003)
Powell, K.J., Brown, P.L., Byrne, R.H., Gajda, T., Hefter, G.T., Sjöberg, S., Wanner, H.: Chemical speciation of environmentally significant metals with inorganic ligands Part 2: The Cu2+–OH−, Cl–, \( {\text{CO}}_{3}^{2 - } \), \( {\text{SO}}_{4}^{2 - } \), and \( {\text{PO}}_{4}^{3 - } \) systems. Pure Appl. Chem. 79, 895–950 (2007)
Wen, J.J.: The fundamental research on removing copper from cobalt electrolyte and nickel electrolyte by ion-exchange with novel silica–polyamine organic–inorganic composite resin. Dissertation, Central South University, Changsha, China (2010)
Chen, X.Y., Chen, A.L., Zhao, Z.W., Liu, X.H., Shi, Y.C., Wang, D.Z.: Removal of Cu from the nickel electrolysis anolyte using nickel thiocarbonate. Hydrometallurgy 133, 106–110 (2013)
Lee, C.I., Yang, W.F., Hsieh, C.I.: Removal of copper(II) by manganese-coated sand in a liquid fluidized-bed reactor. J. Hazard. Mater. 114, 45–51 (2004)
Ramette, R.W.: Copper(II) chloride complex equilibrium constants. Inorg. Chem. 22, 3323–3326 (1983)
Arnek, R., Puigdomenech, I., Valiente, M.: A calorimetric study of copper(II) chloride complexes in aqueous solution. Acta Chem. Scand. A 36, 15–19 (1982)
Byrne, R.H., Van der Weijden, C., Kester, D.R., Zuehlke, R.W.: Evaluation of the CuCl+ stability constant and molar absorptivity in aqueous media. J. Solution Chem. 12, 581–596 (1983)
Moeller, T.: An application of the method of continuous variations to complexion formation in copper(II) salt solutions containing chloride ion. J. Phys. Chem. 48, 111–119 (1944)
Bjerrum, J.: Optical investigations on cupric chloride in mixtures with other chlorides. Mat.-Fys. Medd.-K. Dan Vidensk. Selsk, vol. 22, pp. 1–43 (1946)
Bjerrum, J., Skibsted, L.H.: A contribution to our knowledge of weak chloro complex formation by copper(II) in aqueous chloride solutions. Acta Chem. Scand. A 31, 673–677 (1977)
Bjerrum, J., Skibsted, L.H.: Weak chloro complex formation by copper(II) in aqueous chloride solutions. Inorg. Chem. 25, 2479–2481 (1986)
Bjerrum, J.: Determination of small stability constants. A spectrophotometric study of copper(II) chloride complexes in hydrochloric acid. Acta Chem. Scand. A 41, 328–334 (1987)
Lundstrom, M., Aromaa, J., Forsen, O., Hyvarinen, O., Barker, M.H.: Leaching of chalcopyrite in cupric chloride solution. Hydrometallurgy 77, 89–95 (2005)
Hyvarinen, O., Hamalainen, M.: HydroCopper™—a new technology producing copper directly from concentrate. Hydrometallurgy 77, 61–65 (2005)
Sholz, H., Ludeman, H.D., Franck, E.U.: Spetra of Cu(II)-complexes in aqueous solutions at high temperatures and pressures. Ber. Bunsenges. Phys. Chem. 76, 406–409 (1972)
Helgeson, H.C., Kirkham, D.H., Flowers, G.C.: Theoretical prediction of the thermodynamic behavior of aqueous electrolytes at high pressures and temperatures: IV. Calculation of activity coefficients, osmotic coefficients, and apparent molal and standard and relative partial molal properties to 600 °C and 5 kb. Am. J. Sci. 281, 1249–1516 (1981)
Shriver, D.F., Atkins, P.W., Langford, H.C.: Inorganic Chemistry, 3rd edn. Oxford University Press, Oxford (1996)
Heinrich, C.A., Seward, T.M.: A spectrophotometric study of aqueous iron(II) chloride complexing from 25 to 200 °C. Geochim. Cosmochim. Acta 54, 2207–2221 (1990)
Otakar, S., Petr, N.: Densities of aqueous solutions of inorganic substances. Elsevier, New York (1985)
Zhou, Q.B., Zeng, D., Voigt, W.: Thermodynamic modeling of salt–water systems up to saturation concentrations based on solute speciation: CuCl2–MCl n –H2O at 298 K (M = Li, Mg, Ca). Fluid Phase Equilib. 322–323, 30–40 (2012)
Malinowski, E.R.: Determination of the number of factors and the experimental error in a data matrix. Anal. Chem. 49, 612–616 (1977)
Liu, W., Brugger, J., Mcphail, D.C., Spiccia, L.: A spectrophotometric study of aqueous copper(II)–chloride complexes in LiCl solutions between 100 °C and 250 °C. Geochim. Cosmochim. Acta 66, 3615–3633 (2002)
Hatfield, W.E., Bedon, H.D., Horner, S.M.: Molecular orbital theory for the pentachlorocuprate(II) ion. Inorg. Chem. 4, 1181–1184 (1965)
Migdisov, A.A., Williams-Jones, A.E., Normand, C., Wood, S.A.: A spectrophotometric study of samarium(III) speciation in chloride solutions at elevated temperatures. Geochim. Cosmochim. Acta 72, 1611–1625 (2008)
Suleimenov, O.M., Seward, T.M.: Spectrophotometric measurements of metal complex formation at high temperatures: the stability of Mn(II) chloride species. Chem. Geol. 167, 177–192 (2000)
Uchida, H., Matsuoka, M.: Molecular dynamics simulation of solution structure and dynamics of aqueous sodium chloride solutions from dilute to supersaturated concentration. Fluid Phase Equilib. 219, 49–54 (2004)
Sverjensky, D.A., Shock, E.L., Helgeson, H.C.: Prediction of the thermodynamic properties of aqueous metal complexes to 1000 C and 5 kb. Geochim. Cosmochim. Acta 61, 1359–1412 (1997)
Tagirov, B.R., Zotov, A.V., Akinfiev, N.N.: Experimental study of dissociation of HCl from 350 to 500 °C and from 500 to 2500 bars: thermodynamic properties of HCl0(aq). Geochim. Cosmochim. Acta 61, 4267–4280 (1997)
Migdisov, A.A., Reukov, V.V., Williams-Jones, A.E.: A spectrophotometric study of neodymium(III) complexation in sulfate solutions at elevated temperatures. Geochim. Cosmochim. Acta 70, 983–992 (2006)
Anderson, G.M., Crerar, D.A.: Thermodynamics in Geochemistry: The Equilibrium Model. Oxford University Press, New York (1993)
Helgeson, H.C.: Thermodynamics of hydrothermal systems at elevated temperatures and pressures. Am. J. Sci. 267, 729–804 (1969)
Helgeson, H.C., Kirkham, D.H.: Theoretical prediction of the thermodynamic behavior of aqueous electrolytes at high pressures and temperatures: II. Debye–Hückel parameters for activity coefficients and relative partial molal properties. Am. J. Sci. 174, 1199–1261 (1974)
Kielland, J.: Individual activity coefficients of ions in aqueous solutions. J. Am. Chem. Soc. 59, 1675–1678 (1937)
Pitzer, K.S., Mayorga, G.: Thermodynamics of electrolytes. II. Activity and osmotic coefficients for strong electrolytes with one or both ions univalent. J. Phys. Chem. 77, 2300–2308 (1973)
Pokrovskii, V.A., Helgeson, H.C.: Calculation of the standard partial molal thermodynamic properties of KCl0 and activity coefficients of aqueous KCl at temperatures and pressures to 1000 °C and 5 kbar. Geochim. Cosmochim. Acta 61, 2175–2183 (1997)
Nelder, J.A., Mead, R.: A simplex method for function minimization. Comput. J. 7, 308–313 (1965)
Dennis, J.E., Woods, D.J.: Optimization on microcomputers: The Neld–Mead simplex algorithm. In: Wouk, A. (ed.) New Computing Environments: Microcomputers in Large-Scale Computing, pp. 116–122. SIAM, Philadelphia (1987)
Golub, G.H., Reinsch, C.: Singular value decomposition and least squares solutions. Numer. Math. 14, 403–420 (1970)
Hug, S.J., Sulzberger, B.: In situ Fourier transform infrared spectroscopic evidence for the formation of several different surface complexes of oxalate on TiO2 in the aqueous phase. Langmuir 10, 3587–3597 (1994)
Boily, J.F., Seward, T.M.: Palladium(II) chloride complexation: spectrophotometric investigation in aqueous solutions from 5 to 125 °C and theoretical insight into Pd–Cl and Pd–OH2 interactions. Geochim. Cosmochim. Acta 69, 3773–3789 (2005)
Boily, J.F., Suleimenov, O.M.: Extraction of chemical speciation and molar absorption coefficients with well-posed solutions of Beer’s law. J. Solution Chem. 35, 917–926 (2006)
Liu, W., Etschmann, B., Brugger, J., Spiccia, L., Foran, G., Mcinnes, B.: UV–Vis spectrophotometric and XAFS studies of ferric chloride complexes in hyper-saline LiCl solutions at 25–90 °C. Chem. Geol. 231, 326–349 (2006)
Xia, F.F., Yi, H.B., Zeng, D.: Hydrates of copper dichloride in aqueous solution: a density functional theory and polarized continuum model investigation. J. Phys. Chem. A 113, 14029–14038 (2009)
Xia, F.F., Yi, H.B., Zeng, D.: Hydrates of Cu2+ and CuCl+ in dilute aqueous solution: a density functional theory and polarized continuum model investigation. J. Phys. Chem. A 114, 8406–8416 (2010)
Yi, H.B., Xia, F.F., Zhou, Q.B., Zeng, D.: [CuCl3]− and [CuCl4]2− hydrates in concentrated aqueous solution: a density functional theory and ab initio study. J. Phys. Chem. A 115, 4416–4426 (2011)
Holmes, H.F., Mesmer, R.E.: Thermodynamic properties of aqueous solutions of the alkali metal chlorides to 250 °C. J. Phys. Chem. 87, 1242–1255 (1983)
Yao, Y., Sun, B., Song, P.S., Zhang, Z.: Thermodynamics of aqueous electrolyte solutions isopiestic determination of osmotic and activity coefficients in LiCl–MgCl2–H2O at 25 °C. Acta Chim. Sin. 50, 839–848 (1992)
Tian, Y., Etschmann, B., Liu, W.H., Borg, S., Mei, Y., Testemale, D., O’Neill, B., Rae, N., Sherman, D., Ngothai, Y., Johannessen, B., Glover, C., Brugger, J.: Speciation of nickel(II) chloride complexes in hydrothermal fluids: in situ XAS study. Chem. Geol. 334, 345–363 (2012)
Xia, F.F., Zeng, D., Yi, H., Fang, C.: Direct contact versus solvent-shared ion pairs in saturated NiCl2 aqueous solution: a DFT, CPMD, and EXAFS investigation. J. Phys. Chem. A 117, 8468–8476 (2013)
Acknowledgments
This research was financially supported by the National Natural Science Foundation of China (Nos. 51134007, 2077306) and the China Scholarship Council (No. 201306370118).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, N., Zhou, Q., Yin, X. et al. Trace Amounts of Aqueous Copper(II) Chloride Complexes in Hypersaline Solutions: Spectrophotometric and Thermodynamic Studies. J Solution Chem 43, 326–339 (2014). https://doi.org/10.1007/s10953-014-0129-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-014-0129-8