Abstract
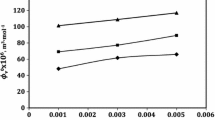
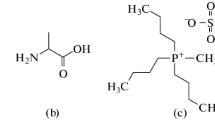
Densities, ρ, and speeds of sound, u, for glycine, l-alanine and l-valine have been measured in (0.2, 0.4, 0.6 and 0.8) mol · kg−1 aqueous solutions of potassium dihydrogen phosphate at temperatures T = (288.15, 293.15, 298.15, 303.15, and 308.15) K. Values of the apparent molar volumes, limiting apparent molar volumes, and transfer volumes have been calculated from the density data. The experimental speeds of sound were used to estimate apparent molar adiabatic compressibilities. The variation of these parameters with temperature is discussed in terms of the role of amino acid and salt in solute–solvent interactions. The UV–visible spectrum has also been used to analyze the results.




Similar content being viewed by others
References
Roth, S.H.: Physical mechanisms of anesthesia. Annu. Rev. Pharmacol. Toxicol. 19, 159–178 (1979)
Frank, N.P., Leib, W.R.: Is membrane expansion relevant to anesthesia? Nature 292, 248–251 (1981)
Marcozzi, G., Correa, N., Luisi, P.L., Caselli, M.: Protein extraction by reverse micelles: a study of the factors affecting the forward and backward transfer of α-chymotrypsin and its activity. Biotechnol. Bioeng. 38, 1239–1246 (1991)
Khoskbarchi, M.K., Vera, J.H.: Reverse micellar extraction and backextraction of l-lysine with three dialkyl sodium phosphinates in pentanol/isooctane mixtures. Sep. Sci. Technol. 30, 2301–2314 (1995)
Von Hippel, P.H., Schleich, T.: In: Timasheff, S.N., Fasman, G.D. (eds.) Structure and Stability of Biological Macromolecules, pp. 417–574. Marcel Dekker, New York (1969)
Jencks, W.P.: Catalysis in Chemistry and Enzymology. McGraw-Hill, New York, p. 351 (1969).
Swenson, D.M., Woolley, E.M.: Apparent molar volume and apparent molar heat capacities of aqueous KI, HIO3, NaIO3 and KIO3 at temperatures from 278.15 K to 393.15 K and the pressure 0.35 MPa. J. Chem. Thermodyn. 40, 54–66 (2008)
Pal, A., Kumar, S.: Volumetric studies of some amino acids in binary aqueous solutions of MgCl2·6H2O at 288 15 and 308 15 K. J. Chem. Sci. 117, 267–273 (2005)
Lark, B.S., Patyar, P., Banipal, T.S., Kishore, N.: Densities, partial molar volumes, and heat capacities of glycine, l-alanine, and l-leucine in aqueous magnesium chloride solutions at different temperatures. J. Chem. Eng. Data 49, 553–565 (2004)
Foroutan, M., Khomami, M.H.: Quaternary (liquid + liquid) equilibria of aqueous two-phase poly (ethylene glycol), poly (DMAM–TBAM), and KH2PO4: experimental and generalized Flory–Huggins theory. J. Chem. Thermodyn. 41, 604–609 (2009)
Nucci, N.V., Vanderkooi, J.M.: Effects of salts of the hofmeister series on the hydrogen bond network of water. J. Mol. Liquids 143, 160–170 (2008)
Kumar, H., Kaur, K., Kumar, S.: Apparent molar volumes and transport behaviour of glycine and l-valine in aqueous solutions of tripotassium citrate at T = (308.15 and 318.15) K. J. Mol. Liquids 162, 89–94 (2011)
Kabiri-Badr, M., Zafarani-Moattar, M.T.: Volumetric and isopiestic studies of (H2O + K2HPO4 + KH2PO4) at 25°C. J. Chem. Eng. Data 40, 412–414 (1995)
Banipal, T.S., Singh, G., Lark, B.S.: Partial molal volumes of transfer of some amino acids from water to aqueous 1,4-dioxane solutions at 298.15 K. Indian J Chem. 39A, 1011–1018 (2000)
Franks, F.: Aqueous Solutions of Amphiphiles and Macromolecules, vol. 4. Plenum Press, New York (1975)
Romero, C.M., Negrete, F.: Effect of temperature on partial molar volumes and viscosities of aqueous solutions of a-dl-Aminobutyric acid, dl-norvaline and dl-norleucine. Phys. Chem. Liq. 42, 261–267 (2004)
Ali, A., Shahjahan, S.: Volumetric and viscometric behaviour of some amino acids and their group contributions in aqueous tetramethylammonium bromide at different temperatures. Z. Phys. Chem. 222, 1519–1532 (2008)
Kumar, D.: Apparent molar volume of some ω-amino acids in aqueous electrolyte systems. Can. J. Chem. 77, 1288–1294 (1999)
Yan, Z., Wang, J.J., Zhang, H., Liu, D.: Volumetric properties of some α-amino acids in aqueous guanidine hydrochloride at 5, 15, 25, and 35°C. J. Solut. Chem. 27, 473–477 (1998)
Zafarani-Moattar, M.T., Sarmad, S.: Effect of tripotassium phosphate on volumetric, acoustic and transport behavior of aqueous solutions of ethyl-3-methylimidazolium bromide at T = (298.15 to 318.15) K. J. Chem. Thermodyn. 42, 1213–1221 (2010)
Frank, H.S., Evans, M.W.: Free volume and entropy in condensed systems. III. Entropy in binary liquid mixtures; Partial molar entropy in dilute solutions; structure and thermodynamics in aqueous electrolytes. J. Chem. Phys. 13, 507–533 (1945)
Lin, G.M., Bian, P.F., Lin, R.S.: The limiting partial molar volume and transfer partial molar volume of glycylglycine in aqueous sodium halide solutions at 298.15 K and 308.15 K. J. Chem. Thermodyn. 38, 144–151 (2006)
Friedman, H.L., Krishnan C.V.: In: Franks, F. (ed.) Water: A Comprehensive Treatise, vol. 3, chap. 1, pp. 1–118. Plenum Press, New York (1973)
Kozak, J.J., Knight, W.S., Kauzman, W.: Solute–solute interactions in aqueous solutions. J. Chem. Phys. 48, 675–690 (1968)
McMillan, W.G., Mayer, J.E.: The statistical thermodynamics of multicomponent systems. J. Chem. Phys. 13, 276–305 (1945)
Friedman, H.L., Krishnan, C.V.: Enthalpies of alkyl sulfonates in water, heavy water, and water-alcohol mixtures and the interaction of water with methylene groups. J. Solut. Chem. 2, 37–51 (1973)
Franks, F., Pedley, M., Reid, D.S.: Solute interactions in dilute aqueous solutions. Part 1–Microcalorimetric study of the hydrophobic interaction. J. Chem. Soc. Faraday Trans. I 72, 359–367 (1976)
Vasantha, T., Attri, P., Venkatesu, P., Rama Devi, R.S.: Thermodynamic contributions of peptide backbone unit from water to biocompatible ionic liquids at T = 298.15 K. J. Chem. Thermodyn. 45, 122–136 (2012)
Vranes, M., Gadzuric, S.B., Zsigrai, I.J., Dozic, S.: Absorption spectra of cobalt(III) chloride and nitrate complexes in aqueous calcium nitrate-ammonium nitrate melts: the influence of solvent composition. J. Mol. Liquids 152, 34–38 (2010)
Acknowledgments
One of the authors (KK) is thankful to the Director and Head, Department of Chemistry, Dr. B. R. Ambedkar, National Institute of Technology, Jalandhar for providing a MHRD fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, H., Kaur, K. Volumetric, Compressibility and UV Spectral Studies on Interactions of Amino Acids with Aqueous Solutions of Potassium Dihydrogen Phosphate at Different Temperatures. J Solution Chem 42, 592–614 (2013). https://doi.org/10.1007/s10953-013-9982-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-013-9982-0