Abstract
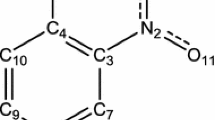
A theoretical study of several para-substituted N-methyl-N-nitrosobenzenesulfonamide biological molecules in MeCN solution has been performed using quantum computational ab initio RHF and density functional B3LYP and B3PW91 methods with the 6-311++G(d,p) basis set. Geometries obtained from DFT calculations were used to perform natural bond orbital analysis. The results show that an intramolecular hydrogen bond exists in the selected molecules, which is confirmed by the NBO analysis. The p characters of the two nitrogen natural hybrid orbitals \( \sigma_{{{\text{N}}3 - {\text{N}}2}} \) increase with increasing \( \sigma_{p} \) values of the para-substituent group on the benzene ring, which results in a lengthening of the N3–N2 bond. It is noted that the weakness of the N–N bond is due to \( n_{{{\text{O}}1}} \to \sigma_{{{\text{N}}3 - {\text{N}}2}}^{*} \) delocalization and is responsible for the longer N3–N2 bond. In addition, there is a direct correlation between hyperconjugation \( n_{{{\text{O}}1}} \to \sigma_{{{\text{N}}3 - {\text{N}}2}}^{*} \) and the bond dissociation energy in the system, which is confirmed by comparison with isoelectronic isomers.

Similar content being viewed by others
References
Thomas, D.D., Ridnour, L.A., Isenberg, J.S., Flores-Santana, W., Switzer, C.H., Donzelli, S., Hussain, P., Vecoli, C., Paolocci, N., Ambs, S., Colton, C.A., Harris, C.C., Roberts, D.D., Wink, D.A.: The chemical biology of nitric oxide: implication in cellular signaling. Free Radic. Biol. Med. 45, 18–31 (2008)
Zhu, X.-Q., Hao, W.-F., Tang, H., Wang, C.-H., Cheng, J.-P.: Determination of N–NO bond dissociation energies of N-methyl-N-nitrosobenzenesulfonamides in acetonitrile and application in the mechanism analyses on NO transfer. J. Am. Chem. Soc. 127, 2696–2708 (2005)
Cheng, J.-P., Wang, K., Yin, Z., Zhu, X.-Q., Lu, Y.: NO affinity. The driving force of nitric oxide (NO) transfer in biomimetic N-nitrosoacetanilide and N-nitrososulfoanilide systems. Tetrahedron Lett. 39, 7925–7928 (1998)
Zhu, X.-Q., He, J.-Q., Li, Q., Ming, X., Lu, J.-M., Cheng, J.-P.: N–NO bond dissociation energies of N-nitroso diphenylamine derivatives (or analogues) and their radical anions: implications for the effect of reductive electron transfer on N–NO bond activation and for the mechanisms of NO transfer to nitranions. J. Org. Chem. 65, 6729–6735 (2000)
Cheng, J.-P., Xian, M., Wang, K., Zhu, X.-Q., Yin, Z., Wang, P.-G.: Heterolytic and homolytic Y–NO bond energy scales of nitroso-containing compounds: chemical origin of NO release and NO capture. J. Am. Chem. Soc. 120, 10266–10267 (1998)
García-Río, L., Leis, J.R., Moreira, J.A., Norberto, F.: Stability and nitrosation efficiency of substituted N-methyl-N-nitrosobenzenesulfonamides. J. Phys. Org. Chem. 11, 756–760 (1998)
García-Río, L., Ramon, L.J., José, M.A., Fatima, N.: Nitrosation and denitrosation of substituted N-methylbenzenesulfonamides. Evidence of an imbalanced concerted mechanism. J. Chem. Soc. Perkin Trans. 2(3), 1613–1620 (1998)
García-Río, L., Ramon, L.J., Jose, M.A., Fatima, N.: Nitroso group transfer from substituted N-Methyl-N-nitrosobenzenesulfonamides to amines. Intrinsic and apparent reactivity. J. Org. Chem. 66, 381–390 (2001)
Moreira, J.A., Rosa da Costa, A.M., García-Río, L., Pessêgo, M.: Equilibrium constants and protonation site for N-methylbenzenesulfonamides. Beilstein J. Org. Chem. 7, 1732–1738 (2011)
Agra, C., Amado, S., Leis, J.R., Rios, A.: Evidence of hydronium ion complexation by 18-crown-6 at the surface of hydrogen dodecyl sulfate micelles. J. Phys. Chem. B 101, 7780–7785 (1997)
Shirlene, M.N., Oh, Y.F., Williams, D.L.H.: Mechanism of S-nitrosation of cysteine derivatives in the pH range 6–12 using N-methyl-N-nitrosotoluene-p-sulphonamide. J. Chem. Soc. Perkin Trans. 2, 755–758 (1989)
Schulz, U., McCalla, D.R.: Reactions of cysteine with N-methyl-N-nitroso-p-toluenesulfonamide and N-methyl-N′-nitro-N-nitrosoguanidine. Can. J. Chem. 47, 2021–2027 (1969)
Garcia, J., González, J., Segura, R., Urpí, F., Vilarrasa, J.: Reaction of. N-nitroso and N-nitro-N-alkylamides with amines. J. Org. Chem. 49, 3322–3327 (1984)
Li, X.-H., Tang, Z.-X., Zhang, X.-Z.: PCM study of bond dissociation energies in N-nitroso compounds. J. Mol. Struct. Theochem 899, 42–45 (2009)
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Zakrzewski, V.G., Montgomery, J.A., Stratmann, R.E., Burant, J.C., Dapprich, S., Millam, J.M., Daniels, A.D., Kudin, K.N., Strain, M.C., Farkas, O., Tomasi, J., Barone, V., Cossi, M., Cammi, R., Mennucci, B., Pomelli, C., Adamo, C., Clifford, S., Ochterski, J., Petersson, G.A., Ayala, P.Y., Cui, Q., Morokuma, K., Malick, D.K., Rabuck, A.D., Raghavachari, K., Foresman, J.B., Cioslowski, J., Ortiz, J.V., Baboul, A.G., Stefanov, B.B., Liu, G., Liashenko, A., Piskorz, P., Komaromi, I., Gomperts, R., Martin, R.L., Fox, D.J., Keith, T., Al-Laham, M.A., Peng, C.Y., Nanayakkara, A., Gonzalez, C., Challacombe, M., Gill, P.M.W., Johnson, B., Chen, W., Wong, M.W., Andres, J.L., Gonzalez, C., Head Gordon, M., Replogle, E.S., Pople, J.A.: GAUSSIAN 03, revision B.02. Gaussian Inc., Pittsburgh, PA (2003)
Becke, A.D.: Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993)
Lee, C., Yang, W., Parr, R.G.: Development of the Colle–Salvetti correlation–energy formula into a functional of the electron density. Phys. Rev. B 37, 785–789 (1988)
Miehlich, B., Savin, A., Stoll, H., Preuss, H.: Results obtained with the correlation energy density functionals of Becke and Lee–Yang and Parr. Chem. Phys. Lett. 157, 200–206 (1989)
Perdew, J.P., Wang, Y.: Accurate and simple analytic representation of the electron–gas correlation energy. Phys. Rev. B. 45, 13244–13249 (1992)
Perdew, J.P.: Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B. 33, 8822–8824 (1986)
Cossi, M., Barone, V., Cammi, R., Tomasi, J.: Ab initio study of solvated molecules: a new implementation of the polarizable continuum model. J. Chem. Phys. Lett. 255, 327–335 (1996)
Glendening, E.D., Reed, A.E., Carpenter, J.E., Weinhold, F.: NBO version 3.1. Gaussian Inc., Pittsburg, PA (2003)
Cao, C.Z.: Substituent effects in organic chemistry. Science Press, Beijing (2003)
Yao, X.-Q., Hou, X.-J., Wu, G.-S., Xu, Y.-Y., Xiang, H.-W., Jiao, H.-J., Li, Y.-W.: Estimation of C–C bond dissociation enthalpies of large aromatic hydrocarbon compounds using DFT methods. J. Phys. Chem. A 106, 7184–7189 (2002)
Luo, Y.R.: Handbook of bond dissociation energies in organic compounds. CRC Press, Boca Raton (2003)
Acknowledgments
We gratefully thank the grant from Development Program in Science and Technology of Henan Province (No. 112300410206) and Henan University of Science and Technology for Young Scholars (No. 2009QN0032) for their support of this work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, XH., Hao, XP., Zhang, XZ. et al. Natural Bond Orbital (NBO) Population Study of Some Para-Substituted N-Methyl-N-nitrosobenzenesulfonamide Biological Molecules in MeCN Solution. J Solution Chem 42, 263–271 (2013). https://doi.org/10.1007/s10953-013-9968-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-013-9968-y