Abstract
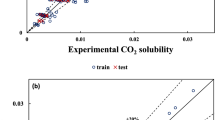
Because of the importance of the solubility of buckminsterfullerene, C60, as the most well-known carbon nanomaterial, a multiparameter linear model is proposed for C60 solubility in different solvents using solvent empirical parameters. The obtained model covers more than 81 and 87 % of the variance in the training and test sets, respectively. On the other hand, because of the potential of solvent empirical parameters for probing different aspects of the solvent–solute interactions, some information about the solubility of C60 in solution phase was obtained. The results showed that hydrogen bond donation ability, basicity scale and dispersion interactions were some of the effective parameters for correlating the solubility of C60 in various solvents.




Similar content being viewed by others
References
Dodziuk, H., et al.: Fullerenes. In: Dodziuk, H. (ed.) Strained Hydrocarbons, pp. 299–315. Wiley, Weinheim (2000)
Liu, H., Tao, G.-h., Evans, D.G., Kou, Y.: Solubility of C60 in ionic liquids. Carbon 43, 1782–1785 (2005)
Beck, M.T., Mándi, G.: Solubility of C60. Fullerene Sci. Technol. 5, 291–310 (1997)
Makitra, R.G., Pristanskii, R.E., Flyunt, R.I.: Solvent effects on the solubility of C60 fullerene. Russ. J. Gen. Chem. 73, 1227–1232 (2003)
Semenov, K.N., Charykov, N.A., Keskinov, V.A., Piartman, A.K., Blokhin, A.A., Kopyrin, A.A.: Solubility of light fullerenes in organic solvents. J. Chem. Eng. Data 55, 13–36 (2010)
Le, T., Epa, V.C., Burden, F.R., Winkler, D.A.: Quantitative structure-property relationship modeling of diverse materials properties. Chem. Rev. 112, 2889–2919 (2012)
Yousefinejad, S., Hemmateenejad, B., Mehdipour, A.R.: New autocorrelation QTMS-based descriptors for use in QSAM of peptides. J. Iran. Chem. Soc. 9, 569–577 (2012)
Yousefinejad, S., Hemmateenejad, B.: A chemometrics approach to predict the dispersibility of graphene in various liquid phases using theoretical descriptors and solvent empirical parameters. Colloids Surf. A: Physicochem. Eng. Asp. (2013). doi:10.1016/j.colsurfa.2013.03.020
Todeschini, R., Consonni, V., Gramatica, P.: Chemometrics in QSAR. In: Brown, S., Walczak, B., Taulér, R. (eds.) Comprehensive Chemometrics, vol. 4, pp. 129–172. Elsevier, Oxford (2009)
Kamlet, M.J., Abraham, M.H., Doherty, R.M., Taft, R.W.: Solubility properties in polymers and biologicalmedia. 4. Correlations of octanol/water partition coefficients with solvatochromic parameters. J. Am. Chem. Soc. 106, 464–466 (1984)
Kamlet, M.J., Doherty, R.M., Carr, P.W., Mackay, D., Abraham, M.H., Taft, R.W.: Linear solvation energy relationships. 44. Parameter estimation rules that allow accurate prediction of octanol/water partition coefficients and other solubility and toxicity properties of polychlorinated biphenyls and polycyclic aromatic hydrocarbons. Environ. Sci. Technol. 22, 503–509 (1988)
Marcus, Y.M., Kamlet, J., Tafts, R.W.: Linear solvation energy relationships. Standard molar Gibbs free energies and enthalpies of transfer of ions from water into nonaqueous solvents. J. Phys. Chem. 92, 3613–3622 (1988)
Hierlemann, A., Zellers, E.T., Ricco, A.J.: Use of linear solvation energy relationships for modeling responses from polymer-coated acoustic-wave vapor sensors. Anal. Chem. 73, 3458–3466 (2001)
Hemmateenejad, B., Safavi, A., Dorostkar, S.: Aggregation of imidazolium based ionic liquids in binary methanol–water solvents: a linear solvation free energy relationship study. J. Mol. Liq. 160, 35–39 (2011)
Toropov, A.A., Leszczynska, D., Leszczynski, J.: QSPR study on solubility of fullerene C60 in organic solvents using optimal descriptors calculated with SMILES. Chem. Phys. Lett. 441, 119–122 (2007)
Gharagheizi, F., Alamdari, R.F.: A molecular-based model for prediction of solubility of C60 fullerene in various solvents. Fullerenes Nanotub. Carbon Nanostruct. 16, 40–57 (2008)
Pourbasheer, E., Riahi, S., Ganjali, M.R., Norouzi, P.: Prediction of solubility of fullerene C60 in various organic solvents by genetic algorithm-multiple linear regression. Fullerenes Nanotub. Carbon Nanostruct. 19, 585–598 (2011)
Liu, H., Yao, X., Zhang, R., Liu, M., Hu, Z., Fan, B.: Accurate quantitative structure-property relationship model to predict the solubility of C60 in various solvents based on a novel approach using a least-squares support vector machine. J. Phys. Chem. B 109, 20565–20571 (2005)
Marcus, Y.: Solubilities of buckminsterfullerene and sulfur hexafluoride in various solvents. J. Phys. Chem. B 101, 8617–8623 (1997)
Kiss, I.Z., Mandi, I., Beck, M.T.: Artificial neural network approach to predict the solubility of C60 in various solvents. J. Phys. Chem. A 104, 8081–8088 (2000)
Kamlet, M.J., Abboud, J.L.M., Taft, R.W.: The solvatochromic comparison method. 6. The π* scale of solvent polarities. J. Am. Chem. Soc. 99, 6027–6038 (1977)
Katritzky, A.R., Tamm, T., Wang, Y., Sild, S., Karelson, M.: QSPR treatment of solvent scales. J. Chem. Inf. Comput. Sci. 39, 684–691 (1999)
Katritzky, A.R., Fara, D.C., Wang, H., Tämm, K., Karelson, M.: Quantitative measures of solvent polarity. Chem. Rev. 104, 175–198 (2004)
Katritzky, A.R., Fara, D.C., Kuanar, M., Hur, E., Karelson, M.: The classification of solvents by combining classical QSPR methodology with principal component analysis. J. Phys. Chem. A 109, 10323–10341 (2005)
Eriksson, L., Jaworska, J., Worth, A.P., Cronin, M.T.D., Mcdowell, R.M., Gramatica, P.: Methods for reliability and uncertainty assessment and for applicability evaluations of classification- and regression-based QSARs. Environ. Health 111, 1361–1375 (2003)
Shao, J.U.N.: Linear model selection by cross-validation. J. Am. Stat. Assoc. 88, 486–494 (2012)
Consonni, V., Ballabio, D., Todeschini, R.: Evaluation of model predictive ability by external validation techniques. J. Chemometr. 24, 194–201 (2010)
Hawkins, D.M., Basak, S.C., Mills, D.: Assessing model fit by cross-validation. J. Chem. Inf. Comput. Sci. 43, 579–586 (2003)
Tetko, I.V., Livingstone, D.J., Luik, A.I.: Neural network studies. 1. Comparison of overfitting and overtraining. J. Chem. Inf. Comput. Sci. 35, 826–833 (1995)
Livingstone, D.J., Salt, D.W.: Judging the significance of multiple linear regression models. J. Med. Chem. 48, 661–663 (2005)
Rücker, C., Rücker, G., Meringer, M.: Y-randomization and its variants in QSPR/QSAR. J. Chem. Inf. Model. 47, 2345–2357 (2007)
Netzeva, T.I., Worth, A.P., Aldenberg, T., Benigni, R., Cronin, M.T., Gramatica, P., Jaworska, J.S., Kahn, S., Klopman, G., Marchant, C.A., Myatt, G., Nikolova-Jeliazkova, N., Patlewicz, G.Y., Perkins, R., Roberts, R., Schultz, T., Stanton, D.W., van de Sandt, J.J., Tong, W., Veith, G., Yang, C.: Current status of methods for defining the applicability domain of (quantitative) structure–activity relationships. ATLA–Altern. Lab. Anim. 33, 1–19 (2005)
Eriksson, L., Jaworska, J., Cronin, M., Worth, A., Gramatica, P., McDowell, R.: Methods for reliability and uncertainty assessment and for applicability evaluations of classification- and regression-based QSARs. Environ. Health Perspect. 111, 1361–1375 (2003)
Craney, T.A., Surles, J.G.: Model-dependent variance inflation factor cutoff values. Quality Eng. 14, 391–403 (2002)
Slinker, B.K., Glantz, S.A.: Multiple regression for physiological data analysis: the problem of multicollinearity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 249, R1–R12 (1985)
Fatemi, M.H., Baher, E., Ghorbanzade’h, M.: Predictions of chromatographic retention indices of alkylphenols with support vector machines and multiple linear regression. J. Sep. Sci. 32, 4133–4142 (2009)
Marcus, Y.: The properties of organic liquids that are relevant to their use as solvating solvents. Chem. Soc. Rev. 22, 409–416 (1993)
Kamlet, M.J., Abboud, J.-L.M., Abraham, M.H., Taft, R.W.: Linear solvation energy relationships. 23. A comprehensive collection of the solvatochromic parameters, π*, α, and β, and some methods for simplifying the generalized solvatochromic equation. J. Org. Chem. 48, 2877–2887 (1983)
Krygowski, T.M., Milcrazek, E., Wrona, P.K.: An extension of the Kamlet–Taft basicity scale of solvents. J. Chem. Soc., Perkin Trans. 2, 1563–1568 (1980)
Kamlet, M.J., Taft, R.W.: Linear solvation energy relationships. Part 1. Solvent polarity–polarizability effects on infrared spectra. J. Chem. Soc., Perkin Trans. 2, 337–341 (1979)
Abraham, M.H., Whiting, G.S., Doherty, R.M., Shuely, W.J.: Hydrogen bonding. Part 13. A new method for the characterization of GLC stationary phases–the Laffort data set. J. Chem. Soc., Perkin Trans. 2, 1451–1460 (1990)
Abraham, M.H., Grellier, P.L., McGill, R.A.: Determination of olive oil–gas and hexadecane–gas partition coefficients, and calculation of the corresponding olive oil–water and hexadecane–water partition coefficients. J. Chem. Soc., Perkin Trans. 2, 797–803 (1987)
Robinson, J.L., Roninson, W.J., Marshall, M.A., Barnes, A.D., Johnson, K.J., Salas, D.S.: Liquid–solid chromatography on amberlite XAD-2 and other styrene–divinylbenzene adsorbents. I. Development of a solvent eluotropic scale. J. Chromatogr. 189, 145–167 (1980)
David, J.G., Hallam, H.E.: Infrared solvent shifts and molecular interactions. Triatomic molecules, carbon disulfide, carbonyl sulfide, and sulfur dioxide. Spectrochim. Acta A 23, 593–603 (1967)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yousefinejad, S., Honarasa, F., Abbasitabar, F. et al. New LSER Model Based on Solvent Empirical Parameters for the Prediction and Description of the Solubility of Buckminsterfullerene in Various Solvents. J Solution Chem 42, 1620–1632 (2013). https://doi.org/10.1007/s10953-013-0062-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-013-0062-2