Abstract
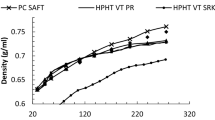
There are numerous models available to compute phase equilibrium composition of supercritical CO2 and H2O at high temperatures and pressures. In this paper a different approach is proposed where liquid state models (LSM) are used following liquid–liquid equilibrium (LLE) flash calculation in order to obtain phase compositions (solubility of CO2 in the H2O rich phase and that of H2O in the CO2 rich phase). Four LSM (two two-parameter models UNIQUAC and LSG, and two three-parameter models NRTL and GEM-RS) are investigated. The original forms of these models are inappropriate to represent the literature values; the binary interaction parameters are related with both pressure and temperature. These modified versions are suitable to generate phase composition values within 2–7 % deviation. Further investigations show that the LLE calculation is more time efficient than vapor–liquid equilibrium computation, meaning our approach can save computational expense for the numerical simulation of CO2 flows in a reservoir. Comparison of the time efficiency of these LSM models with respect to other equations of state is given.



Similar content being viewed by others
References
Climate Change 1995: The science of climate change, summary for policymakers and technical summary of the working group I report. In: Intergovernmental Panel on Climate Change, p. 56. Cambridge, UK (1996)
Climate Change 2001: The scientific basis, summary for policymakers and technical summary of the working group I report. In: Intergovernmental Panel on Climate Change. Cambridge, UK (2001)
Climate change 2007: Mitigation. Contribution of working group III to the fourth assessment report of the intergovernmental panel on climate change. In: Metz, B., Davidson, O.R., Bosch, P.R., Dave, R., Meyer, L.A. (eds.) Intergovernmental Panel on Climate Change. Cambridge, UK and New York, NY, USA (2007)
Gilfillan, S.M.V., Ballentine, C.J., Holland, G., Blagburn, D., Lollar, B.S., Stevens, S., Schoell, M., Cassidy, M.: The noble gas geochemistry of natural CO2 gas reservoirs from the Colorado plateau and Rocky Mountain provinces, USA. Geochimica et Cosmochimica Acta 72, 1174–1198 (2008)
Kaszuba, J.P., Janecky, D.R., Snow, M.G.: Carbon dioxide reaction processes in a model brine aquifer at 200 °C and 200 bars: implications for geologic sequestration of carbon. Appl. Geochem. 18, 1065–1080 (2003)
Pruess, K., Muller, N.: Formation dry-out from CO2 injection into saline aquifers: 1. Effects of solids precipitation and their mitigation. Water Resour. Res. 45, W03402 (2009). doi:10.1029/2008WR007101
Dubessy, J., Buschaert, S., Lamb, W., Pironon, J., Thiery, R.: Methane-bearing aqueous fluid inclusions: Raman analysis, thermodynamic modelling and application to petroleum basins. Chem. Geol. 173, 193–205 (2001)
Roedder, E.: Fluid Inclusions. Reviews in Minerology, vol. 12. Mineralogical Society of America, Washington, DC (1984)
Butcher, S.S., Charlson, R.J., Orians, G.H., Wolfe, G.V.: Global Biogeochemical Cycles. Academic Press, London (1992)
Millero, F.J.: Thermodynamics of the carbon dioxide system in the oceans. Geochim. Cosmochim. Acta 59, 661–677 (1995)
Allen, A.J.: Green Chemistry: Dense Carbon Dioxide and Water as Environmentally Benign Reaction Media. MS Thesis, Massachusetts Institute of Technology, Cambridge (2004)
Fukushima, Y.: Application of supercritical fluids. R&D Rev. Toyota CRDL 3, 1–9 (2000)
Kemmere, M.: Supercritical Carbon Dioxide for Sustainable Polymer Processes. Wiley-VCH, Weinheim (2005)
Ota, M., Abe, Y., Watanabe, M., Smith, R.L.J., Inomata, H.: Methane recovery from methane hydrate using pressurized CO2. Fluid Phase Equilib. 228–229, 553–559 (2005)
Pruess, K.: Enhanced Geothermal Systems (EGS): Comparing Water and CO2 as Heat Transmission Fluids. New Zealand Geothermal Workshop, New Zealand (2007)
White, C.M., Smith, D.H., Jones, K.L., Goodman, A.L., Jikich, S.A., LaCount, R.B., DuBose, S.B., Ozdemir, E., Morsi, B.I., Schroeder, K.T.: Sequestration of carbon dioxide in coal with enhanced coalbed methane recoverys—a review. Energy Fuels 19, 659–724 (2005)
Daridon, J.L., Lagourette, B., Saint-Guirons, H., Xans, P.: A cubic equation of state model for phase equilibrium calculation of alkane + carbon dioxide + water using a group contribution k ij . Fluid Phase Equilib. 91, 31–54 (1993)
Diamond, L.W., Akinfiev, N.N.: Solubility of CO2 in water from −1.5 to 100 °C and from 0.1 to 100 MPa: Evaluation of literature data and thermodynamic modeling. Fluid Phase Equilib. 28, 265–290 (2003)
Duan, Z., Moller, N., Weare, J.H.: Equation of state for the NaCl–H2O–CO2 system: prediction of phase equilibria and volumetric properties. Geochim. Cosmochim. Acta 59, 2869–2882 (1995)
Duan, Z., Sun, R.: An improved model calculating CO2 solubility in pure water and aqueous NaCl solutions from 273 to 533 K and from 0 to 2000 bar. Chem. Geol. 193, 257–271 (2003)
Ferry, J.M., Baumgartner, L.: Thermodynamic models of molecular fluids at the elevated pressures and temperatures of crustal metamorphism. Rev. Miner. Geochem. 17, 323–365 (1987)
Holloway, J.R.: Fugacity and activity of molecular species in supercritical fluids. In: Fraser, D.G. (ed.) Thermodynamics in Geology. D. Reidel Publishing Co., Dordrecht (1977)
Hu, J., Duan, Z., Zhu, C., Chou, I.-M.: PVTx properties of the CO2–H2O and CO2–H2O–NaCl systems below 647 K: assessment of experimental data and thermodynamic models. Chem. Geol. 238, 249–267 (2007)
Kerrick, D.M., Jacobs, G.K.: A modified Redlich–Kwong equation for H2O, CO2, and H2O–CO2 mixtures at elevated pressures and temperatures. Am. J. Sci. 281, 735–767 (1981)
Mader, U.K.: H2O–CO2 mixtures: a review of P-V-T-X data and an assessment from a phase-equilibrium point of view. Can. Miner. 29, 767–790 (1991)
Span, R., Wagner, W.: A new equation of state for carbon dioxide covering the fluid region from the triple-point temperature to 1100 K at pressures up to 800 MPa. J. Phys. Chem. Ref. Data 25, 1509–1596 (1994)
Spycher, N., Pruess, K.: CO2–H2O mixtures in the geological sequestration of CO2. I. Assessment and calculation of mutual solubilities from 12 to 100 °C and up to 600 bar. Geochim. Cosmochim. Acta 67, 3015–3031 (2003)
Spycher, N., Pruess, K.: Mixtures in the geological sequestration of CO2. II. Partitioning in chloride brines at 12–100 °C and up to 600 bar. Geochim. Cosmochim. Acta 69, 3309–3320 (2005)
Tödheide, K.: Hydrothermal solutions. Ber. Bunsenges. Phys. Chem. Geol. 86, 1005–1016 (1982)
Abrams, D.S., Prausnitz, J.M.: Statistical thermodynamics of liquid mixtures: a new expression for the excess Gibbs energy of partly or completely miscible systems. AIChE J. 21, 116–128 (1975)
Renon, H., Prausnitz, J.M.: Local compositions in thermodynamic excess functions for liquid mixtures. AIChE J. 14, 135–144 (1968)
Vera, J.H., Sayegh, S.G., Ratcliff, G.A.: A quasi lattice-local composition model for the excess Gibbs free energy of liquid mixtures. Fluid Phase Equilib. 1, 113–135 (1977)
Keshtkar, A., Jalali, F., Moshfeghian, M.: Evaluation of vapor–liquid equilibrium of CO2 binary systems using UNIQUAC-based Huron–Vidal mixing rules. Fluid Phase Equilib. 140, 107–128 (1997)
Kristensen, J.N., Christensen, P.L.: A combined Soave–Redlich–Kwong and NRTL equation for calculating the distribution of methanol between water and hydrocarbon phases. Fluid Phase Equilib. 82, 199–206 (1993)
Kwak, C., Sandler, S.I., Byun, H.-S.: Correlation of vapor–liquid equilibria for binary mixtures with free energy-based equation of state mixing rules: carbon dioxide with alcohols, hydrocarbons, and several other compounds. Korean J. Chem. Eng. 23(6), 1016–1022 (2006)
Lindeloff, N., Michelsen, M.: Phase envelop calculations for hydrocarbon–water mixtures. SPE J. 8, 298–303 (2003)
Pahlevanzadeh, H., Mohseni-Ahooei, A.: Estimation of UNIQUAC-NRF model parameters for NH3–CO2–H2O system. Iran. J. Chem. Chem. Eng. 24, 21–26 (2005)
Sørensen, H., Pedersen, K.S., Christensen, P.L.: Modeling of gas solubility in brine. Org. Geochem. 33, 635–642 (2002)
Thomsen, K., Rasmussen, P.: Modeling of vapor–liquid–solid equilibrium in gas–aqueous electrolyte systems. Chem. Eng. Sci. 54, 1787–1802 (1999)
Huron, M.-J., Vidal, J.: New mixing rules in simple equations of state for representing vapour–liquid equilibria of strongly non-ideal mixtures. Fluid Phase Equilib. 3, 255–271 (1979)
Wong, D.S., Sandler, S.I.: A theoretically correct equations mixing rule for cubic of state. AIChE J. 38, 671–680 (1992)
Demirel, Y., Gecegormez, H.: Simultaneous correlation of excess Gibbs energy and enthalpy of mixing by the UNIQUAC equation. Can. J. Chem. Eng. 67, 455–461 (1989)
Demirel, Y., Paksoy, H.O., Gecegormez, H.: Correlation of heats of mixing data by the NRTL and UNIQUAC models. Part 2. Predictions of the calorimetric properties. Thermochim. Acta 194, 343–359 (1992)
Nagata, I., Meyer, T., Gmehling, J.: Correlation of binary liquid–liquid equilibrium data over a wide temperature range using UNIQUAC and extended UNIQUAC models. Fluid Phase Equilib. 65, 19–39 (1991)
Skjold-Jorgensen, S., Rasmussen, P., Fredenslund, A.A.: On the temperature dependence of the UNIQUACK/UNIFAC models. Chem. Eng. Sci. 35, 2389–2403 (1980)
Islam, A.W., Kabadi, V.N.: Universal liquid mixture model for vapor–liquid and liquid–liquid equilibria in hexane–butanol–water system over the temperature range 10–100 °C. Chem. Process Eng. 32, 101–115 (2011)
Prausnitz, J.M., Litchtenthaler, R.N., de Azevedo, E.G.: Molecular Thermodynamics of Fluid Phase Equilibria, 3rd edn. Prentice-Hall PTR, Englewood Cliffs (1999)
McPherson, B., Han, W., Cole, B.: Two equation of state assembled for basic analysis of multiphase CO2 flow and in deep sedimentary basin conditions. Comp. Geosci. 34, 427–444 (2008)
Islam, A.W., Javvadi, A., Kabadi, V.N.: Universal liquid mixture models for vapor–liquid and liquid–liquid equilibria in the hexane–butanol–water system. Ind. Eng. Chem. Res. 50, 1034–1045 (2011)
Islam, A.W., Kabadi, V.N.: Analysis of partition coefficients and ternary liquid–liquid equilibrium data for highly non-ideal systems. In: 3rd National Conference of Environmental Science and Technology, pp. 311–317. Greensboro, NC (2007)
Duan, Z., Moller, N., Weare, J.H.: An equation of state for the CH4–CO2–H2O system: I. Pure SYSTEMS from 0 to 1000 °C and 0 to 8000 bar. Geochim. Cosmochim. Acta 56, 2605–2617 (1992)
Islam, A.W., Carlson, E.S.: A fully non-iterative technique for phase equilibrium and density calculations of CO2 + brine system and an equation of state for CO2. In: Proceedings of 37th Stanford Geothermal Workshop, Stanford University (2012)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Islam, A.W., Carlson, E.S. Application of Liquid State Models for the Time Efficient Phase Equilibrium Calculation of Supercritical CO2 and H2O at High Temperatures and Pressures. J Solution Chem 42, 1641–1653 (2013). https://doi.org/10.1007/s10953-013-0052-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-013-0052-4