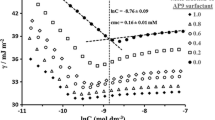
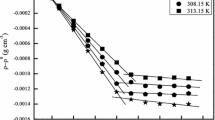
Abstract
Osmotic techniques for measuring thermodynamic activities, such as isopiestic equilibration, are well established for multicomponent solutions, especially mixed salt solutions. Surprisingly, these techniques have not yet been applied to mixed ionic surfactants, despite the numerous practical applications of these systems and the importance of the Gibbs free energy for micelle stability. In this study, mass-action equations are developed for the osmotic coefficients of solutions of ionic surfactant CA + ionic surfactant CB, with common counterion C. Extended Debye–Hückel equations are used for the ionic activity coefficients. The equilibrium constants for mixed micelle formation are evaluated by Gibbs–Duhem integration of critical micelle concentrations. Fitting the derived equations to the osmotic coefficients of aqueous sodium decanoate + sodium dodecylsulfate solutions measured by freezing-point osmometry is used to evaluate the activities of the total surfactant components. Very large departures from ideal solution behavior are indicated, including stoichiometric surfactant activity coefficients and micelle activity coefficients that drop below 0.05 and 10−8, respectively, relative to unity for ideal solutions. Osmometry offers many interesting and unexplored possibilities for studies of mixed surfactant thermodynamics.





Similar content being viewed by others
References
Blandamer, M.J., Engberts, J.B.F.N., Gleeson, P.T., Reis, J.C.R.: Activity of water in aqueous systems; a frequently neglected property. Chem. Soc. Rev. 34, 440–458 (2005)
Elliott, J.A.W., Prickett, R.C., Elmoazzen, H.Y., Porter, K.R., McGann, L.E.: A multisolute osmotic virial equation for solutions of interest in biology. J. Phys. Chem. B 111, 1775–1785 (2007)
Robinson, R.A., Stokes, R.H.: Electrolyte Solutions, 2nd edn. Butterworths, London (1959)
Pitzer, K.S., Brewer, L.: Thermodynamics, 2nd edn. McGraw-Hill, New York (1961). (revised version of 1st edn by Lewis, G. N., Randall, M.)
Pitzer, K.S., Mayorga, G.: Thermodynamics of electrolytes. II. Activity and osmotic coefficients for strong electrolytes with one or both ions univalent. J. Phys. Chem. 77, 2300–2308 (1973)
Burchfield, T.E., Woolley, E.M.: Model for thermodynamics of ionic surfactant solutions. 1. Osmotic and activity coefficients. J. Phys. Chem. 88, 2149–2155 (1984)
McKay, H.A.C., Perring, J.K.: Calculations of the activity coefficients of mixed aqueous electrolytes from vapour pressures. Trans. Faraday Soc. 49, 163–165 (1953)
McKay, H.A.C.: Activities and activity coefficients in ternary systems. Trans. Faraday Soc. 49, 237–242 (1953)
Pan, C.: New forms of McKay–Perring equations. J. Phys. Chem. 72, 2548–2551 (1968)
Pitzer, K.S.: A consideration of Pitzer’s equations for activity and osmotic coefficients in mixed electrolytes. J. Chem. Soc. Faraday Trans. I 80, 3451–3454 (1984)
Yang, J., Pitzer, K.S.: Thermodynamics of electrolyte mixtures. Activity and osmotic coefficients consistent with the higher-order limiting law for symmetrical mixing. J. Solution Chem. 17, 909–924 (1988)
Pitzer, K.S.: Thermodynamics of electrolytes. 1. Theoretical basis and general equations. J. Phys. Chem. 77, 268–277 (1973)
Pitzer, K.S., Kim, J.J.: Thermodynamics of electrolytes. IV. Activity and osmotic coefficients for mixed electrolytes. J. Am. Chem. Soc. 96, 5701–5707 (1974)
Clegg, S.L., Milioto, S., Palmer, D.A.: Osmotic and activity coefficients of aqueous (NH4)2SO4 as a function of temperature, and aqueous (NH4)2SO4–H2SO4 mixtures at 298.15 K. J. Chem. Eng. Data 41, 455–467 (1996)
Rard, J.A., Miller, D.G.: Isopiestic determination for the osmotic and activity coefficients of aqueous mixtures of sodium chloride and magnesium chloride at 25 °C. J. Chem. Eng. Data 32, 85–92 (1987)
Rard, J.A., Clegg, S.L., Platford, R.F.: Thermodynamics of [zNaCl + (1 − z)Na2SO4](aq) from T = 278.15 K to T = 318.15 K, and representation with an extended ion-interaction (Pitzer) model. J. Chem. Thermodyn. 35, 967–1008 (2003)
Dearden, L.V., Woolley, E.M.: Osmotic coefficients of alkyltrimethylammonium bromides in water and aqueous sodium bromide solutions at 55 °C. J. Phys. Chem. 91, 2404–2408 (1987)
Woolley, E.M., Burchfield, T.E.: Thermodynamics of ionic surfactant solutions containing added strong electrolytes. Fluid Phase Equilib. 20, 225–232 (1985)
Widera, B., Neueder, R., Kunz, W.: Vapor pressures and osmotic coefficients of aqueous solutions of SDS, C6TAB, and C8TAB at 25 °C. Langmuir 19, 8226–8229 (2003)
De Lisi, R., Inglese, A., Milioto, S., Pellerito, A.: Demixing of mixed micelles. Thermodynamics of sodium perfluorooctanoate—sodium dodecanoate mixtures in water. Langmuir 13, 192–202 (1997)
De Lisi, R., Inglese, A., Milioto, S., Pellerito, A.: Excess free energy, enthalpy and entropy of surfactant–surfactant mixed micelle formation. Fluid Phase Equilib. 126, 273–287 (1996)
De Lisi, R., Inglese, A., Milioto, S., Pellerito, A.: Thermodynamic studies of sodium dodecyl sulfate–sodium dodecanoate mixtures in water. J. Colloid Interface Sci. 180, 174–187 (1996)
Crisantino, R., De Lisi, R., Milioto, S.: Energetics of sodium dodecylsulfate–dodecyldimethylamine oxide mixed micelle formation. J. Solution Chem. 23, 639–662 (1994)
Kamrath, R.F., Franses, E.I.: Thermodynamics of mixed micellization. Pseudo-phase separation models. Ind. Eng. Chem. 22, 230–239 (1983)
Kamrath, R.F., Franses, E.I.: Mass-action model of micellization. J. Phys. Chem. 88, 1642–1648 (1984)
Maeda, H.: A thermodynamic analysis of charged mixed micelles in water. J. Phys. Chem. B 109, 15933–15940 (2005)
Maeda, H.: A simple thermodynamic analysis of the stability of ionic/nonionic mixed micelles. J. Colloid Interface Sci. 172, 98–105 (1995)
Nagarajan, R., Ruckenstein, R.: Aggregation of amphiphiles as micelles or vesicles in aqueous media. J. Colloid Interface Sci. 71, 580–604 (1979)
Nagarajan, R.: Molecular theory for mixed micelles. Langmuir 1, 331–341 (1985)
Roux, A.H., Hetu, D., Perron, G., Desnoyers, J.E.: Chemical equilibrium model for the thermodynamic properties of mixed aqueous micellar systems: application to thermodynamic functions of transfer. J. Solution Chem. 13, 1–25 (1984)
Peyre, V.: Determination of activities of mixed micelles involving neutral surfactants. Langmuir 18, 1014–1023 (2002)
Scamehorn, J.F. (ed.): Phenomena in Mixed Surfactant Systems, vol. 311. American Chemical Society, Washington, DC (1986)
Clint, J.H.: Micellization of mixed ionic surface active agents. J. Chem. Soc. Faraday Trans. I 71, 1327–1334 (1975)
Holland, P.M., Rubingh, D.N.: Nonideal multicomponent mixed micelle model. J. Phys. Chem. 87, 1984–1990 (1983)
Holland, P.M.: Nonideal mixed micellar solutions. Adv. Colloid Interface Sci. 26, 111–129 (1986)
Holland, P.M., Rubingh, D.N. (eds.): Phenomena in Mixed Surfactant Systems, vol. 501. ACS Symposium SeriesAmerican Chemical Society, Washington, DC (1992)
MacNeil, J.A., Ray, G.B., Leaist, D.G.: Activity coefficients and free energies of nonionic mixed surfactant solutions from vapor-pressure and freezing-point osmometry. J. Phys. Chem. B 115, 5947–5957 (2011)
Sharma, P., MacNeil, J.A., Bowles, J., Leaist, D.G.: The unusual importance of activity coefficients for micelle solutions illustrated by an osmometry study of aqueous sodium decanoate and aqueous sodium decanoate + sodium chloride solutions. Phys. Chem. Chem. Phys. 13, 21333–21343 (2011)
Scatchard, G., Prentiss, S.S.: The freezing points of aqueous solutions. IV. Potassium, sodium and lithium chlorides and bromides. J. Am. Chem. Soc. 55, 4355–4362 (1933)
Hall, D.G.: Electrostatic effects in dilute solutions containing charged colloidal entities. J. Chem. Soc., Faraday Trans. 87, 3529–3535 (1991)
Desnoyers, J.E., Caron, G., De Lisi, R., Roberts, D., Roux, A., Perron, G.: Thermodynamic properties of alkyldimethylamine oxides in water. Application of a mass-action model for micellization. J. Phys. Chem. 87, 1397–1406 (1983)
Philips, J.N.: The energetics of micelle formation. Trans. Faraday Soc. 51, 561–569 (1955)
Benjamin, L.: Calorimetric studies of the micellization of dimethyl-n-alkylamine oxides. J. Phys. Chem. Soc. 68, 3575–3581 (1964)
MacEwan, K., Leaist, D.G.: Quaternary mutual diffusion coefficients for aqueous solutions of a cationic–anionic mixed surfactant from moments analysis of Taylor dispersion profiles. Phys. Chem. Chem. Phys. 5, 3951–3958 (2003)
Wygnal, E., MacNeil, J.A., Bowles, J., Leaist, D.G.: Mutual diffusion with equal eigenvalues in solutions of strongly associated surfactants. A new kind of multicomponent diffusion. J. Mol. Liq. 156, 95–102 (2010)
MacEwan, K., Leaist, D.G.: Incongruent diffusion (negative main diffusion coefficient) for a ternary mixed surfactant system. J. Phys. Chem. B 106, 10296–10300 (2002)
Moulins, J.R., MacNeil, J.A., Leaist, D.G.: Thermodynamic stability and the origins of incongruent and strongly coupled diffusion in solutions of micelles, solubilizates, and microemulsions. J. Chem. Eng. Data 54, 2371–2380 (2009)
Clark, W.M., Rowley, R.L.: Ternary liquid diffusion near Plait points. Int. J. Thermophys. 6, 631–642 (1985)
Acknowledgments
Acknowledgment is made to the Natural Sciences and Engineering Research Council for the financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper is dedicated to Donald G. Miller in recognition and appreciation of his outstanding contributions to research on the transport properties and thermodynamics of solutions.
Rights and permissions
About this article
Cite this article
MacNeil, J.A., Ray, G.B., Sharma, P. et al. Activity Coefficients of Aqueous Mixed Ionic Surfactant Solutions from Osmometry. J Solution Chem 43, 93–108 (2014). https://doi.org/10.1007/s10953-013-0043-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-013-0043-5