Abstract
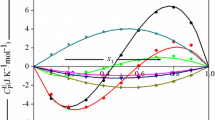
Excess molar volumes, \( V_{123}^{\text{E}} \), of 1, 3-dioxolane (1) + toluene (2) + o- or p-xylene (3) ternary mixtures have been determined dilatometrically over the entire composition range at 298.15 K. For thermodynamic consistency the experimental values were fitted to Redlich–Kister Equation. The \( V_{123}^{\text{E}} \) values of 1, 3-dioxolane (1) + toluene (2) + o- or p-xylene (3) ternary mixtures have been found to be negative over the whole composition range. It has been observed that \( V_{123}^{\text{E}} \) values calculated by graph theory are of the same sign and magnitude with respect to their experimental values.


Similar content being viewed by others
References
Turro, N.J.: Geometric and topological thinking in organic chemistry. Angew. Chem. Int. Ed. 25, 882–901 (1986)
Trinajstic, N.: Chemical Graph Theory, vol. 2. CRC, Boca Raton (1983)
Kier, L.B., Hall, L.H.: Molecular Connectivity in Chemical and Drug Research. Academic Press, London (1976)
Singh, P.P., Nigam, R.K., Singh, K.C., Sharma, V.K.: Topological aspects of the thermodynamics of binary mixtures of non-electrolytes. Thermochim. Acta 46, 175–190 (1981)
Sharma, V.K., Kumar, S.: Topological investigation of molecular interactions in mixtures containing alkanols: Molar excess volumes and molar excess enthalpies. Thermochim. Acta 413, 255–259 (2004)
Kumar, S., Sharma, V.K., Moon, I.: Speed of sound and excess isentropic compressibility of 1,3-dioxolane or 1,4-dioxane + butan-1-ol or butan-2-ol binary mixtures at 308.15 K and atmospheric pressure. Ind. Eng. Chem. Res. 49, 8365–8368 (2010)
Sharma, V.K., Romi: Thermochemical and topological investigations of ternary mixtures containing ether. Indian J. Chem. 40A, 1156–1160 (2001)
Sharma, V.K., Romi, Kumar, S.: Topological investigations of binary and ternary mixtures containing cyclic ether: Excess isentropic compressibility. Indian J. Chem. 42A, 1379–1384 (2003)
Sharma, V.K., Romi, Kumar, S.: Topological investigations of binary and ternary mixtures: Excess isentropic compressibilities. Thermochim. Acta 417, 91–97 (2004)
Kalra, K.C., Sharma, V.K., Katoch, A.: Thermodynamical investigations of some non-electrolytic ternary mixtures. Indian J. Chem. 37A, 308–315 (1998)
Riddick, J.A., Bunger, W.B., Sakano, T.K.: Organic Solvents, Physical Properties and Methods of Purification, vol. II, 4th edn. Wiley-Interscience, New York (1986)
Vogel, A.I.: A Text Book of Practical Organic Chemistry, 5th edn, p. 398. English Book Society and Longman Group, England (2003)
George, J., Sastry, N.V.: Densities, excess molar volumes, viscosities, speed of sound, excess isentropic compressibilities, and relative permittivities for C m H2 m + 1(OCH2CH2) n OH (m = 1 or 2 or 4 and n = 1) + benzene, + toluene, + (o-, m-, and p-) xylenes, + ethylbenzene, and +cyclohexane. J. Chem. Eng. Data 48, 977–989 (2003)
Franscesconi, R., Castellari, C., Comelli, F.: Excess molar enthalpies and excess molar volumes of binary mixtures containing 1,3-dioxolane or 1,4-dioxane + pine resins at (298.15 and 313.15) K and at atmospheric pressure. J. Chem. Eng. Data 46, 577–581 (2001)
Kumar, S., Sharma, V.K., Yadav, J.S., Moon, I.: Thermodynamic investigation of molecular interactions in 1,3-dioxolane or 1, 4-dioxane + benzene or toluene + formamide or N,N-dimethylformamide ternary mixtures at 308.15 K and atmospheric pressure. J. Solution Chem. 39, 680–691 (2010)
Kumaran, M.K., Mc Glashan, M.L.: An improved dilution dilatometer for measurement of excess volumes. J. Chem. Thermodyn. 9, 259–267 (1977)
Redlich, O., Kister, A.T.: Algebraic representation of thermodynamic properties and the classification of solutions. Ind. Eng. Chem. 40, 345–348 (1948)
Katoch, A.: Ph.D. Thesis, Maharishi Dayanand University, Rohtak, India (1998)
Huggins, M.L.: Properties of liquids, including solutions. Part I. Intermolecular energies in monotonic liquids and their mixtures. J. Phys. Chem. 74, 371–378 (1970)
Huggins, M.L.: The thermodynamic properties of liquids, including solutions: Part 2. Polymer solutions considered as diatonic systems. Polymer 12, 389–399 (1971)
Singh, P.P., Bhatia, M.: Energetic of molecular interactions in binary mixtures of non-electrolytes containing a salt. J. Chem. Soc. Faraday Trans. I, 3807–3812 (1989)
Acknowledgments
This study was supported by the Ministry of Education (MOE) of Korea through its BK21 Program and GAS plant R&D Centre funded by the Ministry of Land, Transportation and Maritime Affairs (MLTM) of the Korean Government.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, S., Sharma, V.K. & Moon, I. Volumetric Properties of 1,3-Dioxolane + Toluene + o- or p-Xylene Ternary Mixtures at 25.00 °C and Atmospheric Pressure. J Solution Chem 42, 936–944 (2013). https://doi.org/10.1007/s10953-013-0017-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-013-0017-7