Abstract
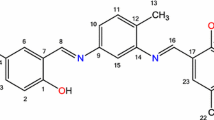
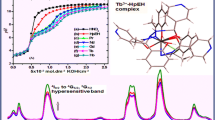
Formation of complexes between the lanthanide ions and N,N′-bis(salicylidene)-4-methyl-1,3-phenylenediamine ligand was studied in solution by pH potentiometry. The potentiometric titration was performed at 25.00 °C in 0.1 mol·dm−3 NaClO4 ionic strength and in DMSO:water (30:70 v:v) solvent mixture. N,N′-bis(salicylidene)-4-methyl-1,3-phenylenediamine ligand (H2L) occurs in three forms: fully or partially deprotonated and unionized. Computer analysis of potentiometric data indicated that in solution the lanthanide (Ln) complexes exist as LnL2, Ln(HL)2 and Ln(H2L)2 species. This observation appears to be in contrast to the solid-state behavior of these complexes prepared in a self-assembly process and structurally defined. Stability constants for La3+, Eu3+, Gd3+, Tb3+, Ho3+ and Lu3+ (Ln3+) complexes were determined. The order of stabilities of LnL2 species in terms of metal ions is La3+ > Eu3+ ≈ Gd3+ = Tb3+ < Ho3+ < Lu3+ with a prominent “gadolinium break”.



Similar content being viewed by others
References
Xie, W.: Formation and crystal structure of a polymeric La(H2salen) complex. Inorg. Chem. 38, 2541–2543 (1999)
Gao, T., Yan, P.-F., Li, G.-M., Hou, G.-F., Gao, J.-S.: Ion size dominated 1D and 2D Salen lanthanide coordination complexes and their luminescence. Polyhedron 26, 5382–5388 (2007)
Bullock, J., Tajmir-Riahi, H-A.: Schiff-base complexes of the lanthanoids and actinoids. Part 1. Lanthanoid(III) halide complexes with the un-ionized form of NN′-ethyl-enebis(salicylideneimine) and related bases. J. Chem. Soc. Dalton Trans. 1, 36–39 (1978)
Yang, X., Jones, R.A., Wong, W.-K.: Anion dependant self-assembly and the first X-ray structure of a neutral homoleptic lanthanide salen complex Tb4(salen)6. Chem. Commun. 28, 3266–3268 (2008)
Yang, X., Jones, R.A.: Anion dependent self-assembly of “tetra-decker” and “triple-decker” luminescent Tb(III) salen complexes. J. Am. Soc. Chem. 127, 7686–7687 (2005)
Lu, Z., Yuan, M., Pan, F., Gao, S., Zhang, D., Zhu, D.: Syntheses, crystal structures, and magnetic characterization of five new dimeric manganese(III) tetradentate Schiff base complexes exhibiting single-molecule–magnet behavior. Inorg. Chem. 45, 3538–3548 (2006)
Rehman, W., Saman, F., Ahmad, I.: Synthesis, characterization, and biological study of some biologically potent Schiff base transition metal complexes. Russ. J. Coord. Chem. 34, 678–682 (2008)
Cozzi, P.G.: Metal–salen Schiff base complexes in catalysis: practical aspects. Chem. Soc. Rev. 33, 410–421 (2004)
Naeimi, H., Safari, J., Heidarnezhad, A.: Synthesis of Schiff base ligands derived from condensation of salicylaldehyde derivatives and synthetic diamine. Dyes Pigm. 73, 251–253 (2007)
Eshtiagh-Hosseini, H., Housaindakht, M.R., Beyramabadi, S.A., Beheshti, S., Esmaeili, A.A., Khoshkholgh, M.J., Morsali, A.: Synthesis, experimental and theoretical characterization of tetra dentate N, N′-dipyridoxyl (1, 3-propylenediamine) salen ligand and its Co(III) complex. Spectrochim. Acta Part A 71, 1341–1347 (2008)
Neelakantan, M.A., Rusalraj, F., Dharmaraja, J., Johnsonraja, S., Jeyakumar, T., Sankaranarayana Pillai, M.: Spectral characterization, cyclic voltammetry, morphology, biological activities and DNA cleaving studies of amino acid Schiff base metal(II) complexes. Spectrochim. Acta Part A 71, 1599–1609 (2008)
Rajabi, F.: A heterogeneous cobalt(II) Salen complex as an efficient and reusable catalyst for acetylation of alcohols and phenols. Tetrahedron Lett. 50, 395–397 (2009)
Kleij, A.W.: Nonsymmetrical salen ligands and their complexes: Synthesis and applications. Eur. J. Inorg. Chem. 2, 193–205 (2009)
Lima, L.F., Corraza, M.L., Cardoza-Filho, L., Màrquez-Alvarez, H., Antunes, O.A.C.: Oxidation of limonene catalyzed by metal(salen) complexes. Braz. J. Chem. Eng. 23, 83–92 (2006)
Papadopoulos, C., Kantiranis, N., Vecchio, S., Lalia-Kantouri, M.: Lanthanide complexes of 3-methoxy-salicylaldehyde thermal and kinetic investigation by simultaneous TG/DTG–DTA coupled with MS. J. Therm. Anal. Calorim. 99, 931–938 (2010)
Benisvy, L., Kannappan, R., Song, Y-F., Milikisyants, S., Huber, M., Mutikainen, I., Turpeinen, V., Gamez, P., Bernasconi, L., Baerends, E.J., Hartl, F., Reedijk, J.: A square-planar nickel(II) monoradical complex with a bis(salicylidene)diamine ligand. Eur. J. Inorg. Chem. 5, 637–642 (2007)
He, J., Yin, Y.-G., Huang, X.-C., Li, D.: Solid structure and photoluminescence of zinc(II) multiplex with heptadentate salicylideneamine as primary ligand. Inorg. Chem. Commun. 9, 205–207 (2006)
Chantarasini, N., Ruangpornvisuti, V., Munangsin, N., Detsen, H., Mananunsp, T., Batiya, C., Chaichit, N.: Structure and physico-chemical properties of hexadentate Schiff base zinc complexes derived from salicylaldehydes and triethylenetetramine. J. Mol. Struct. 701, 93–103 (2004)
Howell, R.C., Spence, K.V.N., Kahwa, I.A., Williams, D.J.: Structure and luminescence of the neutral dinuclear lanthanide(III) complexes [{Ln(api)}2] {H3api = 2-(2-hydroxyphenyl)-1,3-bis[4-(2-hydroxyphenyl)-3-azabut-3-enyl]-1,3-imidazolidine}. J. Chem. Soc. Dalton Trans. 16, 2727–2734 (1998)
Liu, Q., Meermann, C., Görlitzer, H.W., Runte, O., Herdtweck, E., Anwander, P.: Cationic rare-earth metal salen complexes. Dalton Trans. 44, 6170–6178 (2008)
Kaczmarek, M.T., Pospieszna-Markiewicz, I., Kubicki, M., Radecka-Paryzek, W.: Novel lanthanide salicyaldimine complexes with unusual coordination mode. Inorg. Chem. Commun. 7, 1247–1249 (2004)
Radecka-Paryzek, W., Pospieszna-Markiewicz, I., Kubicki, M.: Self-assembled two-dimensional salicylaldimine lanthanum(III) nitrate coordination polymer. Inorg. Chim. Acta 360, 488–496 (2007)
Kaczmarek, M.T., Kubicki, M., Radecka-Paryzek, W.: Self-assembly as a route to dinuclear lanthanide complexes with rare coordination pattern of salen-type ligand. Struct. Chem. 21, 779–786 (2010)
Kaczmarek, M.T, Kubicki, M., Mondry, A., Janicki, R., Radecka-Paryzek, W.: Self-assembled lanthanide salicylaldimines with a unique coordination mode. Eur. J. Inorg. Chem. 14, 2193–2200 (2010)
Irving, M.H., Miles, M.G., Petit, L.D.: The stability constants of some metal chelates of triethylenetetraminehexaacetic acid (ttha). Anal. Chim. Acta 38, 475–488 (1967)
Stańczak, P., Łuczkowski, M., Juszczyk, P., Grzonka, Z., Kozłowski, H.: Interactions of Cu2+ ions with chicken prion tandem repeats. Dalton Trans. 14, 2102–2107 (2004)
Gans, P., Sabatini, A., Vacca, A.: Investigation of equilibria in solution. Determination of equilibrium constants with the HYPERQUAD suite of programs. Talanta 43, 1739–1753 (1996)
Ingri, N., Kakolowicz, W., Sillen, L.G., Warqvist, B.: High-speed computers as a supplement to graphical methods-V1: haltafall, a general program for calculating the composition of equilibrium mixtures. Talanta 14, 1261–1286 (1967)
Łomozik, L., Jaskólski, M., Wojciechowska, A.: A multistage verification procedure for the selection of models in the studies of complex formation equilibria. Pol. J. Chem. 65, 1797–1807 (1991)
Alcock, N.W., Clase, H.J., Willey, G.R., Daly, L.T.: Packing of four independent molecules: 1-methyl-N, N’-bis(salicylidene)-2, 4-phenylenediamine. Acta Cryst. C52, 2340–2343 (1996)
Kaczmarek, M.T., Jastrząb, R., Hołderna-Kędzia, E., Radecka-Paryzek, W.: Self-assembled synthesis, characterization and antimicrobial activity of zinc(II) salicylaldimine complexes. Inorg. Chim. Acta 362, 3127–3133 (2009)
Hernandez-Molina, R., Mederos A., Gili, P., Dominguez S., Lloret F., Cano J., Julve M., Ruiz-Prerz C., Solans X.: Dimer species in dimethyl sulfoxide–water (80:20 w/w) solution of N,N′-bis(salicylideneimine)-m-phenylenediamine (H2sal-m-phen) and similar Schiff bases with CuII, NiII, CoII and ZnII. Crystal structure of [Co2(sal-m-phen)2]·CHCl3. J. Chem. Soc., Dalton Trans. 22, 4327–4334 (1997)
Toraishi, T., Nagasaki, S., Tanaka, S.: Polynuclear complex formation of trivalent lanthanides by 5-sulfosalicylate in an aqueous system—Potentiometric, 1H NMR, and TRLIFS studies. Inorg. Chim. Acta 360, 15751583 (2007)
Gałęzowska, J., Janicki, R., Mondry, A., Burgada, R., Bailly, T., Lecouvey, M., Kozłowski, H.: Coordination ability of trans-cyclohexane-1,2-diamine-N,N,N′,N′-tetrakis(methylenephosphonic acid) towards lanthanide(III) ions. J. Chem. Soc., Dalton Trans., 4384–4394 (2006)
Dash, B.C., Tripathy, P.K., Kanungo, B.K.: Mixed chelates of some trivalent lanthanide ions containing (trans-1, 2-cyclohexylenedinitrilo)tetra-acetate and norleucinate. Monatsh. Chem. 122, 341–348 (1991)
Pardeshi, R.K., Palaskar, N.G., Chondhekar, T.K.: Potentiometric study of lanthanide(III) ion complexes with some Schiff base. J. Indian Chem. Soc. 79, 958–959 (2002)
Pashchevskaya, N.V., Bolotin, S.N., Sokolov, M.E., Sklyar, A.A., Panyushkin, V.T.: Potentiometric study of reactions of rare-earth elements with 3-allylpentanedione in a water–dioxane medium. Russ. J. Gen. Chem. 76, 1011–1014 (2006)
Mahalakshmi Sita, N.: Equilibrium studies of lanthanide(III) complexes of 1-phenyl-3-methyl-4-benzoyl pyrazolone-5 (BMBP) and 1-phenyl-3-methyl-trifluoroacetylpyrazolone-5 (PMTFP). Indian J. Chem. Sec. A, 36A, 118–120 (1997)
Spedding, F.H., Jones, K.C.: Heat capacities of aqueous rare earth chloride solution at 25°. J. Phys. Chem. 70, 2450–2455 (1966)
Spedding, F.H., Csejka, D.A., DeKock, C.W.: Heat of dilution of aqueous rare earth chloride solution at 25°. J. Phys. Chem. 70, 2423–2429 (1966)
Acknowledgments
This work was partially supported by the Polish Ministry of Science and Higher Education (Grant N N204 127 039).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaczmarek, M.T., Jastrząb, R. & Radecka-Paryzek, W. Potentiometric Study of Lanthanide Salicylaldimine Schiff Base Complexes. J Solution Chem 42, 18–26 (2013). https://doi.org/10.1007/s10953-012-9946-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-012-9946-9