Abstract
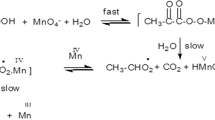
The reaction kinetics for the oxidation of theophylline by permanganate ions have been investigated in perchloric acid medium using spectrophotometric techniques at 25 ∘C, and at constant ionic strength 1.60 mol⋅dm−3, under pseudo first order conditions. An autocatalyzed reaction is observed due to one of the products formed is Mn(II). The orders with respect to theophylline and Mn(VII) were both found to be unity, whereas fractional order is observed with respect to the autocatalyst, Mn(II). The rate of the reaction increases as the concentration of acid increases, but the order with respect to acid concentration is less than unity. The influence of temperature on the rate of reaction was studied. Based on the experimental results a suitable mechanism is proposed. The activation and thermodynamic parameters were determined with respect to slow reaction step.










Similar content being viewed by others
References
Day, M.C., Selbin, J.: Theoretical Inorganic Chemistry, 344 pp. Reinhold Publishing Corporation, New York (1985)
Perez-Benito, J.F.: Autocatalytic reaction pathway on manganese dioxide colloidal particles in the permanganate oxidation of glycine. J. Phys. Chem. 113, 15982–15991 (2009)
Babatunde, O.A.: A study of the kinetics and mechanism of oxidation of L-ascorbic acid by permanganate ion in acid medium. World J. Chem. 3, 27–31 (2008)
Arrizbalaga Sáenz, A., Andres Ordax, F., Fernandez de Aranguiz, M.Y., Pche, R.: Kinetics and mechanism of oxidation of L-α-amino-n-butyric acid by permanganate in acid medium. Int. J. Chem. Kinet. 29, 799–805 (1996)
Arrizbalaga Sáenz, A., Andres Ordax, F., Fernandez de Aranguiz, M.Y., Peche, R.: Kinetic studies on oxidation of amino acids, effect of length of amino acid carbon chain. Int. J. Chem. Kinet. 29, 181–185 (1997)
Wiberg, K.B.: Oxidation in Organic Chemistry, Part A, 48 pp. Academic Press, New York (1965)
Moghadasi, M., Rashidi, N., Iloukhani, H.: Oxidation of L-arginine by manganese(VII) in concentrated sulphuric acid medium. Phys. Chem. Liq. 39, 267–275 (2001)
Surendra Rao, V.B., Sethuram, B.S., Navaneeth Rao, T.: Kinetics of oxidation of some amino acids by KMnO4 in moderately concentrated H2SO4 medium in presence and absence of Ag+. Int. J. Chem. Kinet. 11, 165–173 (1979)
Ernst, T., Cyfert, M.: Kinetics and mechanism of sulphite oxidation by permanganate in acid medium. Z. Phys. Chem. 268, 175–180 (1987)
Purohit, R., Ameta, C.S., Cowdhary, H.C.: Kinetic study of oxidation of tartaric acid by acid permanganate. Z. Phys. Chem. 262, 737–738 (1981)
Solanki, S.K., Khadikar, P.V., Nandwana, R.K.: Kinetic studies in redox reaction of EDTA complex. I. Evidence for free groups in the complex. Z. Phys. Chem. 263, 1069–1071 (1982)
Ameta, S.C., Pande, P.N., Gupta, H.L., Chowdhry, H.C.: Kinetic study of oxidative decarboxylation of L-β-phenylalanine by potassium permanganate. Z. Phys. Chem. 261, 1222–1224 (1980)
Tiwari, P.N., Ameta, S.C., Shastry, V.R.: Kinetics of oxidation of acetic acid by potassium permanganate. Z. Phys. Chem. 260, 167–178 (1979)
Vogel, A.I.: Vogel’s Textbook of Macro and Semimicro Qualitative Inorganic Analysis, 291 pp. Longman, New York (1967)
Timmanagoudar, P.L., Hiremath, G.A., Nandibewoor, S.T.: Permanganate oxidation of thallium(I) in sulphuric acid: a kinetic study by stopped flow technique. Pol. J. Chem. 70, 1459–1467 (1996)
Harihar, A.L., Kembhavi, M.R., Nandibewoor, S.T.: Kinetic and mechanistic study of oxidative deamination and decarboxylation of L-valine by alkaline permanganate. Monatsh. Chem. 13, 739–748 (2000)
Farokhi, S.A., Nandibewoor, S.T.: The kinetic and mechanism of the oxidative decarboxylation of benzilic acid by acid permanganate (stopped flow technique)—autocatalytic study. Can. J. Chem. 82, 1372–1380 (2004)
Chimatadar, S.A., Hiremath, S.C., Raju, J.R.: Oxidation of thallium(I) by permanganate in aqueous perchloric acid medium. Indian J. Chem. 30A, 190–192 (1990)
Bailar, J.C., Emeleus, H.J., Nyholm, R., Dickenson, A.F.T.: Comprehensive Inorganic Chemistry, 771 pp. Pergamon Press Ltd., New York (1975)
Zahedi, M., Bahrami, H.: Kinetic and mechanism of autocatalytic oxidation of L-asperagine in moderately concentrated sulphuric acid medium. Kinet. Catal. 45, 351–358 (2004)
Rajeshwari, H.V., Savanur, A.P., Nandibewoor, S.T., Chimatadar, S.A.: Kinetics and mechanism of uncatalysed and ruthenium(III)-catalysed oxidation of D-panthenol by alkaline permanganate. Transit. Metal Chem. 35, 237–246 (2010)
Shivananda, K.N., Lakshmi, B., Jagdeesh, R.V., Puttaswamy, Mahendra, K.N.: Mechanistic studies on the Ru(III)-catalyzed oxidation of some aromatic primary diamines by chloramines-T in hydrochloric acid medium: a kinetic approach. Appl. Catal. A, Gen. 326, 202–212 (2007)
Jagdeesh, R.V., Puttaswamy: Os(VIII)-catalyzed and uncatalyzed oxidation of biotin by chloramines-T in alkaline medium: comparative mechanistic aspects and kinetic modeling. J. Phys. Org. Chem. 21, 844–848 (2008)
Jyoti, C.A., Shekappa, D.L., Nandibewoor, S.T.: Ruthenium(III) catalyzed oxidative degradation of amitriptyline—a tricyclic anti depressant drug by permanganate in aqueous acidic medium. J. Solution Chem. 40, 502–520 (2011)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hosahalli, R.V., Savanur, A.P., Nandibewoor, S.T. et al. Kinetics and Mechanism of the Autocatalyzed Oxidation of Theophylline by Permanganate in Aqueous Perchloric Acid Medium. J Solution Chem 41, 567–580 (2012). https://doi.org/10.1007/s10953-012-9819-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-012-9819-2