Abstract
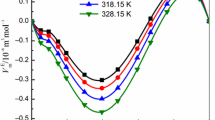
The densities and speeds of sound for binary mixtures containing the solute ionic liquid (IL) methyltrioctylammonium bis(trifluoromethylsulfonyl)imide ([MOA]+[Tf2N]−), solute/solvent methanol, and solvent methyl acetate have been measured at 298.15, 303.15, 308.15 and 313.15 K at atmospheric pressure. The binary mixtures studied are ([MOA]+[Tf2N]− + methyl acetate or methanol), and (methanol + methyl acetate). The apparent molar volume, V φ and the apparent molar isentropic compressibility, k φ , have been evaluated from the experimental density and speed of sound data, respectively. The parameters of a Redlich–Mayer type equation were fitted to the apparent molar volume and apparent molar isentropic compressibility data. The apparent molar volume and apparent molar isentropic compressibility at infinite dilution, \(V_{\phi}^{0}\) and \(k_{\phi}^{0}\), respectively, of the binary solutions have also been calculated at each temperature. The infinite dilution apparent molar volume indicates that intermolecular interactions for (IL + methyl acetate) mixtures are stronger than for (IL + methanol) mixtures at all temperatures except at 298.15 K, and that \(V_{\phi}^{0}\) for the (IL + methyl acetate or methanol) binary systems increases with an increase in temperature. For the (methanol + methyl acetate) system the intermolecular interaction are weaker and \(V_{\phi}^{0}\) also increases with an increase in temperature. Values of the infinite dilution apparent molar expansibility, \(E_{\phi}^{0}\), indicate that the interaction between (IL + methyl acetate) is greater than for (IL + methanol) and (methanol + methyl acetate).
The isentropic compressibilities increase with an increase in temperature for each binary system. At a fixed temperature the isentropic compressibilities also increase with an increase in concentration of the solute for the systems (IL + methyl acetate) and (methanol + methyl acetate), but decrease for the system (IL + methanol). Negative values of \(k_{\phi}^{0}\) for ([MOA]+[Tf2N]− + methyl acetate or methanol) and (methanol + methyl acetate) mixtures can be attributed to the predominance of a penetration effect resulting in greater resistance to compression.
Similar content being viewed by others
References
Hagiwara, R., Ito, Y.: Room temperature ionic liquids of alkylimidazolium cations and fluoroanions. J. Fluorine Chem. 105, 222–227 (2000)
Wong, D.S.H., Chen, J.P., Chang, J.M., Chou, C.H.: Phase equilibria of water and ionic liquids [emim][PF6] and [bmim][PF6]. Fluid Phase Equilib. 194, 1089–1095 (2002)
Freemantle, M.: Designer solvents: ionic liquids may be boost clean technology development. Chem. Eng. News 76, 32–37 (1998)
Seddon, K.R.: Room temperature ionic liquids: neoteric solvents for clean catalysis. Kinet. Catal. 37, 743–748 (1996)
Krummen, M., Wasserscheid, P., Gmehling, J.: Measurement of activity coefficients at infinite dilution in ionic liquids using the dilutor technique. J. Chem. Eng. Data 47, 1411–1417 (2002)
Carmichael, A.J., Seddon, K.R.: Polarity study of some 1-alkyl-3-methylimidazolium ambient temperature ionic liquids with the solvatochromic dye Nile Red. J. Phys. Org. Chem. 13, 591–595 (2000)
Wasserscheid, P., Gordon, C.M., Hilgers, C., Muldoon, M.J., Dunkin, I.R.: Ionic liquids: polar, but weakly coordinating solvents for the first biphasic oligomerisation of ethane to higher α-olefins with cationic Ni complexes. Chem. Commun. 1186–1187 (2001)
Song, C.E., Shim, W.H., Roh, E.J., Lee, S.G., Choi, L.H.: Ionic liquids as powerful media in scandiumtriflate catalysed Diels–Alder reactions: significant rate acceleration, selectivity improvement and easy recycling of catalyst. Chem. Commun. 1122–1123 (2001)
Najdanovic-Visak, V., Esperança, J.M.S., Rebelo, L.P.N.: Phase behaviour of room temperature ionic liquid solutions: an unusually large co-solvent effect in (water + ethanol). Phys. Chem. Chem. Phys. 4, 1701–1703 (2002)
Wheeler, C., West, K.N., Liotta, C.L., Eckert, C.A.: Ionic liquids as catalytic green solvents for nucleophilic displacement reactions. Chem. Commun. 887–888 (2001)
Endres, F.: Electrodeposition of a thin germanium film on gold from a room temperature ionic liquid. Chem. Phys. 3, 3165–3174 (2001)
Wasserscheid, P., Keim, W.: Ionic liquids—new “solutions” for transition metal catalysis. Angew. Chem., Int. Ed. Engl. 39, 3772–3789 (2000)
Kato, R., Gmehling, J.: Activity coefficients at infinite dilution of various solutes in the ionic liquids [MMIM]+[CH3SO4]−, [MMIM]+[CH3OC2H4SO4]−, [MMIM]+[(CH3)2PO4]−, [C5H5NC2H5]+[(CF3SO2)2N]− and [C5H5NH]+[C2H5OC2H4OSO3]−. Fluid Phase Equilib. 226, 37–44 (2004)
Martyn, J.E., Kenneth, R.S.: Ionic liquids. Green solvent for future. Pure Appl. Chem. 72, 1391–1398 (2000)
Heintz, A., Kulikov, D.V., Verevkin, S.P.: Thermodynamic properties of mixtures containing ionic liquids. Activity coefficients at infinite dilution of polar solutes in 4-methyl-N-butyl-pyridinium tetrafluoroborate using gas-liquid chromatography. J. Chem. Thermodyn. 34, 1314–1347 (2002)
Heintz, A., Kulikov, D.V., Verevkin, S.P.: Thermodynamic properties of mixtures containing ionic liquids. 2. Activity coefficients at infinite dilution of hydrocarbons and polar solutes in 1-methyl-3-ethyl-imidazolium bis(trifluoromethyl-sulfonyl) amide and in 1,2-dimethyl-3-ethyl-imidazolium bis(trifluoromethyl-sulfonyl) amide using gas-liquid chromatography. J. Chem. Eng. Data 47, 894–899 (2002)
Letcher, T.M., Deenadayalu, N., Soko, B., Ramjugernath, D., Nevines, A., Naicker, P.K.: Activity coefficients at infinite dilution of organic solutes in 1-hexyl-3-methylimidazolium hexafluorophosphate from gas-liquid chromatography. J. Chem. Eng. Data 48, 708–711 (2003)
Letcher, T.M., Soko, B., Reddy, P., Deenadayalu, N.: Determination of activity coefficients at infinite dilution of solutes in the ionic liquid 1-hexyl-3-methylimidazolium tetrafluoroborate using gas-liquid chromatography. J. Chem. Eng. Data 48, 1587–1590 (2003)
Vasiltsova, T.V., Verevkin, S.P., Bich, E., Heintz, A., Bogel-Lukasik, R., Domańska, U.: Thermodynamic properties of mixtures containing ionic liquids. Activity coefficients of ethers and alcohols in 1-methyl-3-ethyl-imidazolium bis(trifluoromethyl-sulfonyl) imide using the transpiration method. J. Chem. Eng. Data 50, 142–148 (2005)
Kato, R., Krummen, M., Gmehling, J.: Measurement and correlation of vapour–liquid equilibria and excess enthalpies of binary systems containing ionic liquids and hydrocarbons. Fluid Phase Equilib. 224, 47–54 (2004)
Marsh, K.N., Deev, A., Wu, A.C.-T., Tran, E., Klamt, A.: Room temperature ionic liquids as replacements for conventional solvents—a review. Korean J. Chem. Eng. 19, 357–362 (2002)
Najdanovic-Visak, V., Esperança, J.M.S.S., Rebelo, L.P.N., da Ponte, M.N., Guedes, H.J.R., Seddon, K.R., de Souza, H.C., Szydlowski, J.: Pressure, isotope, and water co-solvent effects in liquid–liquid equilibria of (ionic liquid + alcohol) systems. J. Phys. Chem. B 107, 12797–12807 (2003)
Heintz, A., Lehmann, J.K., Wertz, C.: Thermodynamic properties of mixtures containing ionic liquids. 3. Liquid–liquid equilibria of binary mixtures of 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide with propan-1-ol, butan-1-ol, and pentan-1-ol. J. Chem. Eng. Data 48, 472–474 (2003)
Crosthwaite, J.M., Aka, S.N.V., Magin, E.J., Brennecke, J.F.: Liquid phase behavior of imidazolium-based ionic liquids with alcohols. J. Phys. Chem. B 108, 5113–5119 (2004)
Wu, C.-T., Marsh, K.N., Deev, A.V., Boxall, J.A.: Liquid–liquid equilibria of room-temperature ionic liquids and butan-1-ol. J. Chem. Eng. Data 48, 486–491 (2003)
Domańska, U., Marciniak, A.: Solubility of 1-alkyl-3-methylimidazolium hexafluorophosphate in hydrocarbons. J. Chem. Eng. Data 48, 451–456 (2003)
Domańska, U., Marciniak, A.: Solubility of ionic liquid [emim][PF6] in alcohols. J. Phys. Chem. B 108, 2376–2382 (2004)
Letcher, T.M., Deenadayalu, N.: Ternary liquid–liquid equilibria for mixtures of 1-methyl-3-octyl-imidazolium chloride + benzene + an alkane at T=298.2 K and 1 atm. J. Chem. Thermodyn. 35, 67–76 (2003)
Crosthwaite, J.M., Aki, S.N.V., Maginn, E.J., Brennecke, J.F.: Liquid phase behaviour of imidazolium-based ionic liquids with alcohols: effect of hydrogen bonding and non-polar interactions. Fluid Phase Equilib. 228–229, 303–309 (2005)
Domańska, U., Bogel-Lukasik, E., Bogel-Lukasik, R.: Solubility of 1-dodecyl-3-methylimidazolium chloride in alcohols (C2–C12). J. Phys. Chem. B 107, 1858–1863 (2003)
Domańska, U., Bogel-Lukasik, E.: Measurements and correlation of the (solid + liquid) equilibria of [1-decyl-3-methylimidazolium chloride + alcohols (C2–C12)]. Ind. Eng. Chem. Res. 42, 6986–6992 (2003)
Domańska, U., Mazurowska, L.: Solubility of 1,3-dialkylimidazolium chloride or hexafluorophosphate or methylsulfonate in organic solvents. Effect of the anions on solubility. Fluid Phase Equilib. 221, 73–82 (2004)
Sadeghi, R., Shekaari, H., Hosseini, R.: Effect of alkyl chain length and temperature on the thermodynamic properties of ionic liquids 1-alkyl-3-methylimidazolium bromide in aqueous and non-aqueous solutions at different temperatures. J. Chem. Thermodyn. 41, 273–289 (2009)
Zafarani-Moattar, M.T., Shekaari, H.: Apparent molar volume and isentropic compressibility of ionic liquid 1-butyl-3-methylimidazolium bromide in water, methanol, and ethanol at T = (298.15 to 318.15) K. J. Chem. Thermodyn. 37, 1029–1035 (2005)
Das, D., Das, B., Hazra, D.K.: Ultrasonic velocities and isentropic compressibilities of some symmetrical tetraalkylammonium salts in N,N-dimethylacetamide at 298.15 K. J. Mol. Liq. 111, 15–18 (2004)
Radhamma, M., Venkatesu, P., Prabhakara Rao, M.V., Lee, M.-J., Lin, H.-M.: Excess molar volumes and ultrasonic studies of dimethylsulphoxide with ketones at T=303.15 K. J. Chem. Thermodyn. 40, 492–497 (2008)
Pires, R.M., Costa, H.F., Ferreira, A.G.M., Fonseca, I.M.A.: Viscosity and density of water + ethyl acetate + ethanol mixtures at 298.15 and 318.15 K and atmospheric pressure. J. Chem. Eng. Data 52, 1240–1245 (2007)
Rohman, N., Dass, N.N., Mahiuddin, S.: Isentropic compressibility of aqueous and methanolic sodium thiocyanate solutions. J. Chem. Eng. Data 44, 465–472 (1999)
Wahab, A., Mahiuddin, S.: Isentropic compressibility, electrical conductivity, shear relaxation time, surface tension, and Raman spectra of aqueous zinc nitrate solutions. J. Chem. Eng. Data 49, 126–132 (2004)
Abraham, R., Abdulkhadar, M., Asokan, C.V.: Ultrasonic investigation of molecular interaction in binary mixtures of nitriles with methanol/toluene. J. Chem. Thermodyn. 32, 1–16 (2000)
Raju, K., Rajamannan, B., Rakkappan, C.: Ultrasonic study of molecular interactions in binary mixtures of aprotic and inert solvents. J. Mol. Liq. 100, 113–118 (2002)
Galan, J.J., Del Castillo, J.L., Gonzalez-Perez, A., Czapkiewicz, J., Rodriguez, J.R.: Density and sound velocity studies of aqueous solutions of tetradecyltrimethylammonium nitrate at different temperatures. J. Solution Chem. 32, 919–927 (2003)
Blandmer, M.J., Davis, M.I., Douheret, G., Reis, J.C.: Apparent molar isentropic compressions and expansions of solutions. Chem. Soc. Rev. 30, 8–15 (2001)
Sibiya, P.N., Deenadayalu, N.: Excess molar volumes and isentropic compressibility of binary systems {trioctylmethylammonium bis(trifluoromethylsulfonyl) imide + methanol or ethanol or 1-propanol} at different temperatures. J. Chem. Thermodyn. 40, 1041–1045 (2008)
Deenadayalu, N., Kumar, S., Bhujrajh, P.: Liquid densities and excess molar volumes for (ionic liquids + methanol + water) ternary system at atmospheric pressure and at various temperatures. J. Chem. Thermodyn. 39, 1318–1324 (2007)
Bhujrajh, P., Deenadayalu, N.: Liquid densities and excess molar volumes for binary systems (ionic liquid + methanol or water) at 298.15, 303.15 and 313.15 K, and at atmospheric pressure. J. Solution Chem. 36, 563–672 (2007)
Deenadayalu, N., Ngcongo, K.C., Letcher, T.M., Ramjugernath, D.: Liquid–liquid equilibria for ternary mixtures (an ionic liquid + benzene + heptane or hexadecane) at T=298.2 K and atmospheric pressure. J. Chem. Eng. Data 51, 988–991 (2006)
Deenadayalu, N., Thango, S.H., Letcher, T.M., Ramjugernath, D.: Measurement of activity coefficients at infinite dilution using polar and non-polar solutes in the ionic liquid 1-methyl-3-octyl-imidazolium diethyleneglycolmonomethylethersulfate at T = (288.15, 298.15, and 313.15) K. J. Chem. Thermodyn. 38, 542–546 (2006)
Deenadayalu, N., Bhujrajh, P.: Density, speed of sound, and derived thermodynamic properties of ionic liquids [EMIM]+ [BETI]− or ([EMIM]+[CH3(OCH2CH2)2OSO3]− + methanol or + acetone) at T = (298.15 or 303.15 or 313.15) K. J. Chem. Eng. Data 53, 1098–1102 (2008)
Oswal, S.L., Putta, S.S.R.: Excess molar volumes of binary mixtures of alkanols with ethyl acetate from 298.15 to 323.15 K. Thermochim. Acta 373, 141–152 (2001)
Hasan, M., Hiray, A.P., Kadam, U.B., Shirude, D.F., Kurhe, K.J., Sawant, A.B.: Densities, sound speed, and IR studies of (methanol + 1-acetoxybutane) and (methanol + 1,1-dimethylethyl ester) at (298.15, 303.15, 308.15, and 313.15) K. J. Chem. Eng. Data 55, 535–538 (2010)
Oswal, S.L., Oswal, P., Modi, P.S., Dave, J.P., Gardas, R.L.: Acoustic, volumetric, compressibility and refractivity properties and Flory’s reduction parameters of some homologous series of alkyl alkanoates from 298.15 to 333.15 K. Thermochim. Acta 410, 1–14 (2004)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bahadur, I., Deenadayalu, N. Apparent Molar Volume and Isentropic Compressibility for the Binary Systems {Methyltrioctylammonium Bis(trifluoromethylsulfonyl)imide + Methyl Acetate or Methanol} and (Methanol + Methyl Acetate) at T=298.15, 303.15, 308.15 and 313.15 K and Atmospheric Pressure. J Solution Chem 40, 1528–1543 (2011). https://doi.org/10.1007/s10953-011-9740-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-011-9740-0