Abstract
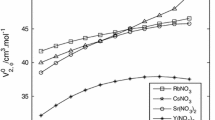
For the first time cesium diffusivity in aqueous solutions of rubidium chloride is being reported here in the concentration range from 0.001 to 4.00 mol⋅dm−3. The measurement use a radioactive tracer technique employing a sliding cell mechanism. These diffusivity values were utilized to understand the transport mechanism of Cs ion in the RbCl–H2O system using the Onsager-Gosting-Harned equation and the extended Debye-Hückel equation. The observed deviation between the theoretical and experimental diffusivities are explained by introducing the concept of Field-Dielectric-Gradient forces and energies that exist around an ion, which takes care of the finite size of the ion, ion-water interaction and the ion-ion interaction in a continuum basis.
Similar content being viewed by others
References
Kropman, M.K., Bakkar, H.J.: Dynamics of water molecules in aqueous solvation shells. Science 291, 2118–2120 (2001)
Chandra, A.: Effects of ion atmosphere on hydrogen-bond dynamics in aqueous electrolyte solutions. Phys. Rev. Lett. 85, 768–771 (2000)
Barthel, J.M.G., Krienke, H., Kunz, W.: Physical Chemistry of Electrolyte Solutions. Springer, New York (1998)
Hubbard, J.B., Wolynes, P.G.: In: Dogonadze, R.R., et al. (eds.) Chemical Physics of Solvation Part D. Elsevier, Amsterdam (1988)
Roux, B., Karplus, M.: Molecular dynamics simulations of the gramicidin channel. Ann. Rev. Biophys. Biomol. Struct. 23, 732–753 (1994)
Chakrabarti, H.: Cation diffusion coefficients in CsCl–H2O system over the concentration range 0.009 to 10.00 mol⋅dm−3 at 25 °C. Appl. Radiat. Isot. 45, 171–175 (1994)
Rusli, I.T., Schrader, G.L., Larson, M.A.: Raman spectroscopic study of NaNO3 solution system–solute clustering in supersaturated solution. J. Cryst. Growth 97, 345–351 (1989)
Georgalis, Y., Kierzek, A.M., Saenger, W.: Cluster formation in aqueous electrolyte solutions observed by dynamic light scattering. J. Phys. Chem. B 104, 3405–3406 (2000)
Ruanhui, L., Leaist, D.G.: Mutual diffusion in solutions of alkali metal halides–aqueous LiF, NaF and KF at 25 °C. J. Chem. Soc. Faraday Trans. 94, 111–114 (1998)
Rajurkar, N.S., Patil, D.D.: Electrolyte diffusion of cesium bromide in water at 25 °C. Appl. Radiat. Isot. 55, 289–292 (2001)
Rajurkar, N.S., Gokarn, N.A.: Studies on self and electrolyte diffusion in cesium halides. Appl. Radiat. Isot. 58, 441–445 (2003)
Dufreche, J.F., Bernard, O., Turq, P.: Transport of electrolyte solutions: are ions Brownian particles? J. Mol. Liq. 118, 189–194 (2005)
Behzadi, B., Patel, B.H., Galindo, A., Ghotbi, C.: Modeling electrolyte solutions with the SAFT–VR equation using Yukawa potentials and the mean–spherical approximation. Fluid Phase Equilib. 236, 241–255 (2005)
Goa, G.-H., Shi, H.-B., Yu, Y.-X.: Mutual diffusion coefficients of concentrated 1:1 electrolyte from the modified meas spherical approximation. Fluid Phase Equilib. 256, 105–111 (2007)
Haghtalab, A., Mazloumi, S.H.: A square-well equation of state for aqueous strong electrolyte solutions. Fluid Phase Equilib. 285, 96–104 (2009)
Hasan, S.A.: Morphology of ion clusters in aqueous electrolytes. Phys. Rev. E 77, 031501 (2008)
Mills, R., Woolf, L.A.: Tracer–diffusion coefficients of cesium ion in aqueous alkali chloride solutions at 25 °C. J. Phys. Chem. 63, 2068–2069 (1959)
Stell, G., Patey, G.N., Hoye, J.S.: Dieletric constants of fluid models: statistical mechanical theory and its quantitative implementation. Adv. Chem. Phys. 48, 183–328 (1981)
Ramanathan, P.S., Friedman, H.L.: Study of a refined model for aqueous 1–1 electrolytes. J. Chem. Phys. 54, 1086–1099 (1971)
Friedman, H.L.: Ionic Solution Theory. Interscience, New York (1963)
Duferche, J.F., Bernard, O., Turq, P., Mukherjee, A., Bagchi, B.: Ionic self diffusion in concentrated aqueous electrolyte solutions. Phys. Rev. Lett. 88, 095902 (2002)
Friedman, A.M., Kennedy, J.W.: The self-diffusion coefficients of potassium, cesium, iodide and chloride ions in aqueous solutions. J. Am. Chem. Soc. 77, 4499–4501 (1955)
Chakrabarti, H., Changdar, S.N.: Accurate measurement of tracer diffusion coefficient in aqueous solutions with sliding cell technique. Appl. Radiat. Isot. 43, 405–417 (1992)
Chakrabarti, H.: Strong evidence of isotope effect in diffusion of NaCl and CsCl solution. Phys. Rev. B 51, 12809–12812 (1995)
Chakrabarti, H.: Anomalies in the ion transport of phosphoric acid in water and heavy water environments. J. Phys. Cond. Matt. 8, 7019–7029 (1996)
Gosting, L.J., Harned, H.S.: The application of Onsager theory of ionic mobilities to self-diffusion. J. Am. Chem. Soc. 73, 159–161 (1951)
Onsager, L., Fuoss, R.M.: Irreversible processes in electrolytes. Diffusion, conductance, and viscous flow in arbitrary mixtures of strong electrolytes. J. Phys. Chem. 36, 2689–2778 (1930)
Stokes, R.H., Woolf, L.A., Mills, R.A.: Tracer diffusion of iodide ion in aqueous alkali chloride solutions at 25 °C. J. Am. Chem. Soc. 61, 1634–1636 (1957)
Bahe, L.W.: Structure in concentrated solutions. Field–dielectric–gradient forces and energies. J. Phys. Chem. 76, 1062–1071 (1972)
Ritson, D.M., Hasted, J.B.: Dielectric properties of aqueous ionic solutions. Parts I & II. J. Chem. Phys. 16, 1–21 (1948)
Padova, J.: Ion–solvent interaction. II. Partial molar volume and electrostriction: a thermodynamic approach. J. Chem. Phys. 39, 1552–1557 (1963)
Hyman, A., Vaughn, P.A.: Small angle scattering by solutions of complex ions. In: Proceeding of the Conference on “Small Angle Scattering” held at Syracuse Univ., June 1965, Gordon and Breach New York, N. Y., 1967, p. 477
Mills, R., Lobo, V.M.M.: Physical Sciences Data 36: Self Diffusion in Electrolyte Solutions. Elsevier, Amsterdam (1989)
Chakrabarti, H., Sil, S., Kundu, S.: (to be published)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chakrabarti, H., Kanjilal, B. Measurement of the Diffusivity of Cesium Ion in Aqueous Rubidium Chloride Solution. J Solution Chem 39, 409–416 (2010). https://doi.org/10.1007/s10953-010-9508-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-010-9508-y