Abstract
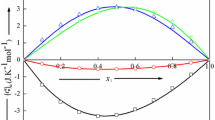
Experimental molar heat capacity data (Cp m) and excess molar heat capacity data (\(\mathit{Cp}^{\mathrm{E}}_{\mathrm{m}}\)) of binary mixtures containing water + (formamide or N,N-dimethylformamide or dimethylsulfoxide or N,N-dimethylacetamide or 1,4-dioxane) at several compositions, in the temperature range 288.15 K to 303.15 K and atmospheric pressure, have been determined using a modified 1455 PAAR solution calorimeter. The excess heat capacities are positive for aqueous solutions containing 1,4-dioxane, N,N-dimethylformamide or dimethylsulfoxide, negative for solutions containing water + formamide and show a sigmoid behavior for mixtures containing water + N,N-dimethylacetamide, over the whole composition range. The experimental excess molar heat capacities are discussed in terms of the influence of temperature and of the organic solvent type present in the binary aqueous mixtures, as well as in terms of the existing molecular interactions and the organic solvent’s molecular size and structure.
Similar content being viewed by others
References
Torres, R.B., Marchiore, A.C.M., Volpe, P.L.O.: Volumetric properties of binary mixtures of (water + organic solvents) at temperatures between T=288.15 K and T=303.15 K at p=0.1 MPa. J. Chem. Therm. 38, 526–541 (2006)
Scharlin, P., Steinby, K., Domanska, U.: Volumetric properties of binary mixtures of N,N-dimethylformamide with water or water-D2 at temperatures from 277.13 K to 318.15 K. J. Chem. Therm. 34, 927–957 (2002)
Zaichikov, A.M.: Thermochemical study of the ternary system water–formamide–N,N-dimethylacetamide. Russ. J. Gen. Chem. 71, 162–167 (2001)
Jelinska-Kazimierczuk, M., Szydlowski, J.: Physicochemical properties of solutions of amides in H2O and in D2O. J. Solution Chem. 30, 623–640 (2001)
Lin, R., Hu, X., Ren, X.: Homogeneous enthalpic interaction of amino acids in DMF-H2O mixed solvents. Thermochim. Acta 352–353, 31–37 (2000)
Sacco, A., Matteoli, E.: Isotopic substitution effects on the volumetric and viscosimetric properties of water–dimethylsulfoxide mixtures at 25 °C. J. Solution Chem. 26, 527–535 (1997)
Miyai, K., Nakamura, M., Tamura, K., Murakami, S.: Isotope Effects on thermodynamic properties in four binary systems: water (or heavy water) + dimethylsulfoxide (or N,N-dimethylformamide) at 25 °C. J. Solution Chem. 26, 973–987 (1997)
Singh, P.P., Raj, B.: Thermodynamics of 1:1 electrolyte mixtures in aquo-1,4-dioxane solvent system: molar excess enthalpy. Thermochim. Acta 230, 113–122 (1993)
Visser, C., Heuvelsland, W.J.M., Dunn, L.A., Somsen, G.: Some properties of binary aqueous liquid mixtures. J. Chem. Soc. Faraday Trans. 74, 1159–1169 (1978)
Zaichikov, A.M., Manin, N.G.: Thermochemical study of solvation of aliphatic carboxiamides in aqueous formamide solutions. Russ. J. Gen. Chem. 71, 679–688 (2001)
Suzuki, T., Fujisawa, M., Takagi, S., Kimura, T.: Excess enthalpies of water +1,4-dioxane at 278.15, 298.15, 318.15 and 338.15 K. J. Therm. Anal. Calorim. 85, 545–550 (2006)
Zaichikov, A.M., Krestov, G.A.: Thermodynamic properties of the water–N,N-dimethylformamide system. Russ. J. Phys. Chem. 69, 353–357 (1995)
Tamura, K.: Excess heat capacities of the mixtures containing methylcyclohexane at 298.15 K. Fluid Phase Equilibr. 182, 303–312 (2001)
Nishimoto, M., Tamura, T., Murakami, S.: Excess isobaric heat capacity, density and speed of sound of {xCF3CH2OH+(1−x)CH3CN} at the temperature 308.15 K. J. Chem. Therm. 29, 15–22 (1997)
Piñeiro, A.: Excess volumes and isobaric heat capacities of diisopropil ether with alkanols at 298.15 K: Application of the symmetrical extended real associated solution model. Fluid Phase Equilibr. 216, 245–256 (2004)
Hanks, R.W., Christensen, J.J.: The prediction of multi component vapor-liquid equilibria from binary heats of mixing. I. Chem. Symp. Ser. 56, 31–48 (1980)
Murty, A.K.S., Zudkevitch, D.: Effect of heat of mixing and vapor-liquid equilibrium on design, performance and economics of distillation. I. Chem. Symp. Ser. 56, 51–77 (1980)
Lide, D.R.: Handbook of Chemistry and Physics, 86th edn. CRC, Boca Raton (2007)
Checoni, R.F., Francesconi, A.Z.: Measurement and correlation of excess molar enthalpy at various temperatures − acetonitrile + diethylamine or s-butylamine mixtures. J. Therm. Anal. Calorim. 80, 295–301 (2005)
Rubini, K., Francesconi, R., Bigi, A., Comelli, F.: Excess molar enthalpies and heat capacities of dimethylsulfoxide + seven normal alkanols at 303.15 K and atmospheric pressure. Thermochim. Acta 452, 124–127 (2007)
Dean, J.A.: Lange’s Handbook of Chemistry, 13th edn. McGraw-Hill, New York (1985)
Egan, E.P., Luff Jr., B.B., B, B.: Heat of solution, heat capacity and density of aqueous solutions at 25 °C. J. Chem. Eng. Data 11, 194–196 (1966)
Schoemaker, D.P., Garland, C.W., Nibler, J.W.: Experiments in Physical Chemistry, 5th edn. McGraw-Hill, New York (1982)
Clemett, C.J.: Nuclear magnetic resonance study of water dissolved in some cyclic ethers. J. Chem. Soc. A, Inorg. Phys. Theor. 455–458 (1969)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Checoni, R.F., Volpe, P.L.O. Measurements of the Molar Heat Capacities and Excess Molar Heat Capacities for Water + Organic Solvents Mixtures at 288.15 K to 303.15 K and Atmospheric Pressure. J Solution Chem 39, 259–276 (2010). https://doi.org/10.1007/s10953-010-9500-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-010-9500-6