Abstract
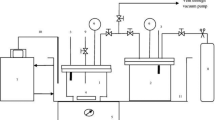
Despite its success for modeling electrolyte thermodynamics in aqueous media, the use of the Pitzer approach for the investigation of electrolytes in nonaqueous or in mixed solvent media is still very limited. Further, a review of the literature reveals that there are no more than a few research groups who have used the exact form of the Pitzer–Simonson–Clegg (PSC) ion-interaction approach for the investigation of electrolytes in mixed solvent systems. As a continuation of our previous studies, the present investigation reports modeling of HCl in the 2-propanol + water mixed solvent system with the Pitzer, PSC and an extended form of the PSC ion-interaction approaches using the experimental potentiometric data from a cell containing pH glass membrane and Ag/AgCl electrodes. The electrochemical measurements were performed over the HCl molality range from 0.01 to 4.5 mol⋅kg−1 in mixed 2-propanol (x%)+water (100−x%) solvents, with different solvent percent mass fractions (x%=10,20,30,40 and 50%) at 298.15±0.05 K.
Similar content being viewed by others
References
Pitzer, K.S.: Thermodynamics of electrolytes. I. Theoretical basis and general equations. J. Phys Chem. 77, 268–277 (1973)
Pitzer, K.S., Mayorga, G.: Thermodynamics of electrolytes. II. Activity and osmotic coefficients for strong electrolytes with one or both ions univalent. J. Phys. Chem. 77, 2300–2308 (1973)
Pitzer, K.S., Kim, J.J.: Thermodynamics of electrolytes. IV. Activity and osmotic coefficients for mixed electrolytes. J. Am. Chem. Soc. 96, 5701–5707 (1974)
Pitzer, K.S. (ed.): Activity Coefficients in Electrolyte Solutions, 2nd edn. CRC, Boca Raton (1991)
Chen, C., Britt, H., Boston, J., Evans, L.: Local composition model for the excess Gibbs energy of aqueous electrolyte systems. AIChE J. 28, 588–596 (1982)
Renon, H.: Models for excess properties of electrolyte solutions: molecular bases and classification, needs and trends for new developments. Fluid Phase Equilib. 116, 217–224 (1996)
Haghtalab, A., Vera, J.: A nonrandom factor model for the excess Gibbs energy of electrolyte solutions. AIChE J. 34, 803–813 (1988)
Marshall, S., May, P., Hefter, G.: Least square analysis of osmotic coefficient data at 25 °C according to Pitzer’s equation, 1:1 electrolytes. J. Chem. Eng. Data 40, 1041–1052 (1995)
Perez-Villasenor, F., Iglesias-Silva, G.A., Hall, K.R.: Osmotic and activity coefficients using a modified Pitzer equation for strong electrolytes 1:1 and 1:2 at 298.15 K. Ind. Eng. Chem. Res. 41, 1031–1037 (2002)
Das, B.: Thermodynamics of electrolytes in mixed solvent media. Application of the Pitzer ion interaction approach. Can. J. Chem. 83, 2032–2038 (2005)
Pitzer, K.S., Simonson, J.M.: Thermodynamics of multicomponent, miscible, ionic systems: theory and equations. J. Phys. Chem. 90, 3005–3009 (1986)
Simonson, J.M., Pitzer, K.S.: Thermodynamics of multicomponent, miscible, ionic systems: the system LiNO3–KNO3–H2O. J. Phys. Chem. 90, 3009–3013 (1986)
Clegg, S.L., Pitzer, K.S.: Thermodynamics of multicomponent, miscible, ionic solutions: Generalized equations for symmetrical electrolytes. J. Phys. Chem. 96, 3513–3520 (1992)
Clegg, S.L., Pitzer, K.S., Brimblecombe, P.: Thermodynamics of multicomponent, miscible, ionic solutions. 2. Mixtures including unsymmetrical electrolytes. J. Phys. Chem. 96, 9470–9479 (1992)
Hu, Y.F., Guo, T.M.: Thermodynamics of electrolytes in aqueous systems containing both ionic and nonionic solutes. Application of the Pitzer–Simonson–Clegg equations to activity coefficients and solubilities of 1:1 electrolytes in four ternary systems at 298.15 K. Phys. Chem. Chem. Phys. 1, 3303–3308 (1991)
Ferreira, L.A., Macedo, E.A., Pinho, S.P.: Effect of KCl and Na2SO4 on the solubility of glycine and DL-alanine at 298.15 K. Ind. Eng. Chem. Res. 44, 8892–8898 (2005)
Ferreira, L.A., Macedo, E.A., Pinho, S.P.: KCl Effect on the solubility of five different amino acids in water. Fluid Phase Equilib. 255, 131–137 (2007)
Clegg, S.L., Seinfeld, J.H., Brimblecombe, P.: Thermodynamic modelling of aqueous aerosols containing electrolytes and dissolved organic compounds. Aerosol Sci. 32, 713–738 (2001)
Li, Y.G., Mather, A.E.: Correlation and prediction of the solubility of CO2 and H2S in aqueous solutions of triethanolamine. Ind. Eng. Chem. Res. 35, 4804–4809 (1996)
Li, Y.G., Mather, A.E.: Correlation and prediction of the solubility of CO2 and H2S in aqueous solutions of methyldiethanolamine. Ind. Eng. Chem. Res. 36, 2760–2765 (1997)
Li, Y.G., Mather, A.E.: Correlation and prediction of the solubility of CO2 and H2S in an aqueous solution of 2-piperidineethanol and sulfolane. Ind. Eng. Chem. Res. 37, 3098–3104 (1998)
Kundu, M., Mandal, B.P., Bandyopadhyay, S.S.: Vapor–liquid equilibrium of CO2 in aqueous solutions of 2-amino-2-methyl-1-propanol. J. Chem. Eng. Data 48, 789–796 (2003)
Kundu, M., Bandyopadhyay, S.S.: Solubility of CO2 in water + diethanolamine + 2-amino-2-methyl-1-propanol. J. Chem. Eng. Data 51, 398–405 (2006)
Kundu, M., Bandyopadhyay, S.S.: Solubility of CO2 in water + diethanolamine + N-methyldiethanolamine. Fluid Phase Equilib. 248, 158–167 (2006)
Harned, H.S., Calmon, C.: The properties of electrolytes in mixtures of water and organic solvents. I. hydrochloric acid in ethanol- and isopropanol-water mixtures of high dielectric constant. J. Am. Chem. Soc. 61, 1491–1494 (1939)
Roy, R.N., Vernon, W., Bothwell, A.L.M.: Thermodynamics of hydrochloric acid in 1-propanol–water mixtures from emf measurements at different temperatures. Electrochim. Acta 18, 81–85 (1973)
Bates, R.G.: Determination of pH: Theory and Practice. Wiley, New York (1964)
Deyhimi, F., Salamat-Ahangari, R., Ghalami-Choobar, B.: Determination of activity coefficients of NH4Cl in methanol-water mixed solvents at 25 °C by electromotive force measurements. Phys. Chem. Liq. 41, 605–611 (2003)
Deyhimi, F., Ebrahimi, A., Roohi, H., Koochaki, K.: Determination of activity coefficients, osmotic coefficients, and excess Gibbs free energies of HCl in N,N-dimethylformamide-water mixed solvent systems by potentiometric measurements. J. Chem. Eng. Data 49, 1185–1188 (2004)
Deyhimi, F., Salamat-Ahangari, R.: Potentiometric investigation of the thermodynamic properties of the ternary mixed (NH4Cl + CaCl2+H 2O) electrolyte system. Fluid Phase Equilib. 264, 113–121 (2008)
Zielen, A.J.: The elimination of liquid junction potentials with the glass electrode. J. Phys. Chem. 67, 1474–1479 (1963)
Esteso, M.A., Gonzalez-Diaz, O.M., Hernandez-Luis, F.F., Fernandez-Merida, L.: Activity coefficients for NaCl in ethanol-water mixtures at 25 °C. J. Solution Chem. 18, 277–288 (1989)
Gonzalez-Diaz, O.M., Fernandez-Merida, L., Hernandez-Luis, F.F., Esteso, M.A.: Activity coefficients for NaBr in ethanol-water mixtures at 25 °C. J. Solution Chem. 24, 551–563 (1995)
Garcia-Paneda, E., Maestre, A., Yanes, C.: Activity coefficients for NaCl in (w)N,N-dimethylformamide + (1−w) water mixtures at 25 °C. J. Chem. Eng. Data 44, 1055–1057 (1999)
Harned, H.S., Owen, B.B.: The Physical Chemistry of Electrolytic Solutions, 3rd edn. Reinhold, New York (1958)
Sakurai, M.: Partial molar volumes in aqueous mixtures of nonelectrolytes. II. Isopropyl alcohol. J. Solution Chem. 17, 267–275 (1988)
Gupta, A.R.: Thermodynamics of electrolytes in mixed solvents. Application of Pitzer’s thermodynamic equations to activity coefficients of 1:1 electrolytes in methanol-water mixtures. J. Phys. Chem. 83, 2986–2990 (1979)
Oiwa, I.T.: The activity coefficients of hydrochloric acid in methanol-water mixtures. J. Phys. Chem. 60, 754–759 (1956)
Koh, D.S.P., Khoo, K.H., Chan, C.Y.: The application of the Pitzer equations to 1−1 electrolytes in mixed solvents. J. Solution Chem. 14, 635–651 (1985)
Hamer, W.J., Wu, Y.C.: Osmotic coefficients and mean activity coefficients of uni-univalent electrolytes in water at 25 °C. J. Phys. Chem. Ref. Data 1, 1047–1099 (1972)
Marzal, P., Monto, J.B., Rodrigo, M.A.: Isobaric vapor-liquid equilibria of the water + 2-Propanol system at 30, 60, and 100 kPa. J. Chem. Eng. Data 41, 608–611 (1996)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Deyhimi, F., Karimzadeh, Z. Pitzer and Pitzer–Simonson–Clegg Modeling Approaches: Ternary HCl + 2-Propanol + Water Electrolyte System. J Solution Chem 39, 245–257 (2010). https://doi.org/10.1007/s10953-010-9497-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-010-9497-x