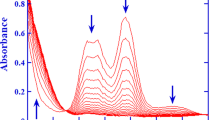
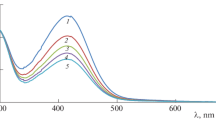
Abstract
Fursemide is the chemical compound 4-chloro-2-(furan-2-ylmethylamino)-5-(aminosulfonyl) benzoic acid. It was oxidized by diperiodatocuprate(III) in alkali solutions, and the oxidation products were identified as furfuraldehyde and 2-amino-4-chloro-5-(aminosulfonyl) benzoic acid. The reaction kinetics were studied spectrophotometrically. The reaction was observed to be first order in [oxidant] and fractional order each in [fursemide] and [periodate], whereas added alkali retarded the rate of reaction. The reactive form of the oxidant was inferred to be [Cu(H3IO6)2]−. A mechanism consistent with the experimental results was proposed, in which oxidant interacts with the substrate to give a complex as a pre-equilibrium state. This complex decomposed in a slow step to give a free radical that was further oxidized by reaction with another molecule of DPC to yield 2-amino-4-chloro-5-(aminosulfonyl) benzoic acid and furfuraldehyde in a fast step. This reaction was studied at 25, 30, 35, 40 and 45 °C, and the activation parameters E a,ΔH #,ΔS # and ΔG # were determined to be 51 kJ⋅mol−1,48.5 kJ⋅mol−1,−63.5 J⋅K−1⋅mol−1 and 67 kJ⋅mol−1, respectively. The value of log 10 A was calculated to be 6.8.
Similar content being viewed by others
References
Aventis Pharma: Lasix Approved Product Information. Aventis Pharma Pty Ltd, Lane Cove (1998)
Rossi, S.: Australian Medicines Handbook. Australian Medicines Handbook Pty Ltd, Adelaide (2004)
Korpi, E.R., Kuner, T., Seeburg, P.H., Luddens, H.: Selective antagonist for the cerebellar granule cell-specific gamma-aminobutyric acid type A receptor. Mol. Pharmacol. 47, 283–289 (1995)
Kulkarni, S.D., Nandibewoor, S.T.: A kinetic and mechanistic study on oxidation of isoniazid drug by alkaline diperiodatocuprate(III)—a free radical intervention. Transit. Met. Chem. 31, 1034–1039 (2006)
Sethuram, B.: Some Aspects of Electron Transfer Reactions Involving Organic Molecules, p. 71. Allied Publishers (P) Ltd, New Delhi (2003)
Jose, T.P., Tuwar, S.M.: Oxidation of threonine by the analytical reagent diperiodatocuprate(III)—an autocatalysed reaction. J. Mol. Struct. 827, 137–144 (2007)
Jaiswal, P.K., Yadava, K.L.: Determination of sugars and organic acids with periodate complex of Cu(III). Indian J. Chem. 11, 837–838 (1973)
Jeffery, G.H., Bassett, J.M., Mendham, J., Denny, R.C.: Vogel’s Text Book of Quantitative Chemical Analysis, 5th edn. ELBS, Longman, Harlow (1996), pp. 371–455
Sharanabasamma, K., Salunke, M.S., Tuwar, S.M.: Periodate influencing diperiodatocuprate(III) oxidation of sulphur containing amino acid in aqueous alkaline medium—an induced reaction. J. Solution Chem. 37, 1217–1225 (2008)
Vogel’s Organic Qualitative Analysis. Pearson Education, Delhi (2006)
Nomura, Y.: Organic spot tests I. Detection of Acidic Compounds. Short Commun. 32(5), 536 (1959)
Feigl, F.: Spot Tests in Organic Analysis, p. 444. Elsevier, New York (1975)
Lide, D.R.: CRC Handbook of Chemistry and Physics, 73rd edn., pp. 8–51. CRC Press, London (1992)
Kolthoff, I.M., Meehan, E.J., Carr, E.M.: Mechanism of initiation of emulsion polymerization by persulfate. J. Am. Chem. Soc. 75, 1439–1441 (1953)
Crouthamel, C.E., Meck, H.V., Martin, D.S., Banks, C.V.: Spectrophotometric studies of dilute aqueous periodate solutions. J. Am. Chem. Soc. 73, 82–87 (1951)
Lister, M.W.: The stability of some complexes of trivalent copper. Can. J. Chem. 31, 638–652 (1953)
Sethuram, B.: Some Aspects of Electron Transfer Reactions Involving Organic Molecules, p. 77. Allied Publishers (P) Ltd, New Delhi (2003)
Bal Reddy, K., Sethuram, B., Navaneeth Rao, T.: Kinetics of oxidative deamination and decarboxylation of some amino acids by diperiodato cuprate(III) in alkaline medium. Indian J. Chem. A 20, 395–397 (1981)
Haines, R.I., McAuley, A.: Synthesis and reactions of nickel(III) complexes. Coord. Chem. Rev. 39, 77–119 (1981)
Tuwar, S.M., Nandibewoor, S.T., Raju, J.R.: Oxidation of allyl alcohol by diperiodatonickelate(IV) in aqueous alkaline medium. J. Indian Chem. Soc. 69, 651–653 (1992)
Jose, T.P., Angadi, M.A., Salunke, M.S., Tuwar, S.M.: Oxidation of atenolol by diperiodatoargentate(III) in aqueous alkaline medium—a multimechanistic reaction. Main Group Chem. 7, 109–122 (2008)
Kumar, A., Kumar, P., Ramamurthy, P.: Kinetics of oxidation of glycine and related substrates by diperiodatoargentate(III). Polyhedron 18, 773–777 (1999)
Mayerstein, D.: Trivalent copper. I. Pulse radiolytic study of the chemical properties of the aquo complex. Inorg. Chem. 10, 638–641 (1971)
Laidler, K.J.: Chemical Kinetics, 3rd edn. Pearson Education, Delhi (2004).
Weissberger, A.: Investigation of Rates and Mechanism of Reactions in Techniques of Chemistry, 4th edn., p. 421. Wiley-Interscience, New York (1974)
Rangappa, K.S., Anita, N., Made Gowda, N.M.: Mechanistic study of oxidation of substituted phenethyl alcohols by manganese(III) sulfate catalyzed by ruthenium(III) ion in acid medium. Synth. React. Inorg. Met.-Org. Chem. 31, 1499–1518 (2001)
Puttaswamy, Anuradha, T.M.: Kinetic analysis of oxidation of dopamine by sodium N-chlorobenzene sulphamide in perchloric acid media: a mechanistic approach. Indian J. Chem. A 40, 514–518 (2001)
Walling, C.: Free Radicals in Solution. Wiley, New York (1957)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Angadi, M.A., Tuwar, S.M. Oxidation of Fursemide by Diperiodatocuprate(III) in Aqueous Alkaline Medium—a Kinetic Study. J Solution Chem 39, 165–177 (2010). https://doi.org/10.1007/s10953-009-9492-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-009-9492-2