Abstract
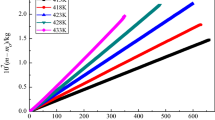
The molar enthalpies of solution of an alanine-based ionic liquid (IL) [C4mim][Ala], 1-butyl-3-methylimidazolium alanine, containing various amount of water and various molalities Δsol H m(wc), were measured with a solution-reaction isoperibol calorimeter at (298.15±0.01) K, where wc denotes water content. According to Archer’s method, the standard molar enthalpies of solution of [C4mim][Ala] containing known amounts of water, \(\Delta_{\mathrm{sol}}H_{\mathrm{m}}^{\mathrm{o}}(\mathrm{wc})\) , were obtained. In order to eliminate the effect of the small amount of residual water in the source [C4mim][Ala], a linear fitting of \(\Delta_{\mathrm{sol}}H_{\mathrm{m}}^{\mathrm{o}}(\mathrm{wc})\) against water content was carried out, yielding a good straight line where the intercept is the standard molar enthalpy of solution of anhydrous [C4mim][Ala], \(\Delta_{\mathrm{sol}}H_{\mathrm{m}}^{\mathrm{o}}(\mathrm{pure}\ \mathrm{IL})=-(61.42\pm 0.08)\) kJ⋅mol−1. The hydration enthalpy of the alanine anion [Ala]− was estimated using Glasser’s lattice energy theory.
Similar content being viewed by others
References
Tao, G.H., He, L., Sun, N., Kou, Y.: New generation ionic liquids: cations derived from amino acids. Chem. Commun. (Camb.) 3562–3564 (2005). doi:10.1039/b504256a
Tao, G.H., He, L., Liu, W.-S., Xu, L., Xiong, W., Wang, T., Kou, Y.: Preparation, characterization and application of amino acid-based green ionic liquids. Green Chem. 8, 639–646 (2006). doi:10.1039/b600813e
Fukumoto, K., Ohno, H.: New generation ionic liquids: cations derived from amino acids. Chem. Commun. (Camb.) 3081–3083 (2006). doi:10.1039/b606613e
Fukumoto, K., Yoshizawa, M., Ohno, H.: New generation ionic liquids: cations derived from amino acids. J. Am. Chem. Soc. 127, 2398–2399 (2005). doi:10.1021/ja043451i
Ohno, H., Fukumoto, K.: Amino acid ionic liquids. Acc. Chem. Res. 40, 1122–1129 (2007). doi:10.1021/ar700053z
Zhang, Z.-F., Li, J.-G., Zhang, Q.-G., Guan, W., Yang, J.-Z.: Enthalpy of solution of amino acid ionic liquid 1-ethyl-3-methylimidazolium ammonioacetate. J. Chem. Eng. Data 53, 1196–1198 (2008). doi:10.1021/je700599z
Guan, W., Xue, W.-F., Li, N., Tong, J.: The enthalpy of solution of amino acid ionic liquid 1-butyl-3-methylimidazolium glycine. J. Chem. Eng. Data 53, 1401–1403 (2008). doi:10.1021/je800067m
Fang, D.-W., Guan, W., Tong, J., Wang, Z.-W., Yang, J.-Z.: Study on physico-chemical properties of ionic liquids based on alanine [C n mim][Ala] (n=2,3,4,5,6). J. Phys. Chem. B 112, 7499–7505 (2008). doi:10.1021/jp801269u
Yang, J.-Z., Zhang, Q.-G., Wang, B., Tong, J.: Study on the properties of amino acid ionic liquid EMIGly. J. Phys. Chem. B 110, 22521–22524 (2006). doi:10.1021/jp0648691
Najdanovic-Visak, V., Ksperanca, J.M.S.S., Rebelo, L.P.N., Da Ponte, M.N., Guedes, H.J.R., Seddon, K.R., Sydlowski, J.: Phase behaviour of room temperature ionic liquid solution: an unusually large co-solvent effect in (water + ethanol). Phys. Chem. Chem. Phys. 4, 1701–1703 (2002). doi:10.1039/b201723g
Tong, J., Hong, M., Guan, W., Yang, J.-Z.: Studies on the thermodynamic properties of new ionic liquids: 1-methyl-3-pentylimidazolium salts containing metal of group III. J. Chem. Thermodyn. 38, 1416–1421 (2006). doi:10.1016/j.jct.2006.01.017
Rogers, R.D., Seddon, K.R.: Ionic Liquids as Green Solvents. ACS Symposium Series, vol. 856. American Chemical Society, Washington (2003)
Yang, J.-Z., Tong, J., Li, J.-B.: Study of the volumetric properties of the aqueous ionic liquid 1-methyl-3-pentylimidazolium tetrafluoroborate. J. Solution Chem. 36, 573–582 (2007). doi:10.1007/s10953-007-9134-5
Yang, J.-Z., Guan, W., Tong, J., Wang, H., Li, L.: Studies on thermo-chemical properties of a new ionic liquid prepared by mixing 1-methyl-3-pentylimidazolium chloride with InCl3. J. Solution Chem. 35, 845–852 (2006). doi:10.1007/s10953-006-9028-y
Archer, D.G., Widegren, J.A., Kirklin, D.R., Magee, J.W.: Enthalpy of solution of 1-octyl-3-methylimidazolium tetrafluoroborate in water and in aqueous sodium fluoride. J. Chem. Eng. Data 50, 1484–1491 (2005). doi:10.1021/je050136i
Glasser, L.: Lattice and phase transition thermodynamics of ionic liquids. Thermochim. Acta 421, 87–93 (2004). doi:10.1016/j.tca.2004.03.015
Wilkes, J.S., Levisky, J.A., Wilson, R.A.: Dialkylimidazolium chloroaluminate metal: a new class of room-temperature ionic liquids for electrochemistry, spectroscopy, and synthesis. Inorg. Chem. 21, 1263–1264 (1982). doi:10.1021/ic00133a078
Di, Y.Y., Qu, S.S., Liu, Y., Wen, D., Tang, C.H., Li, L.W.: A thermochemical study of the solid-state coordination reactions of two α-amino acids with copper(II) acetate. Thermochim. Acta 387, 115–119 (2002). doi:10.1016/S0040-6031(01)00831-0
Yu, H.G., Liu, Y., Tan, Z.C., Dong, J.X., Zou, T.J., Huang, X.M., Qu, S.S.: A solution-reaction isoperibol calorimeter and standard molar enthalpies of formation of Ln(hq)2Ac (Ln = La, Pr). Thermochim. Acta 401, 217–224 (2003). doi:10.1016/S0040-6031(02)00566-X
Rychly, R., Pekarek, V.: The use of potassium chloride and tris(hydroxymethyl) aminomethane as standard substances for solution calorimetry. J. Chem. Thermodyn. 9, 391–396 (1977). doi:10.1016/0021-9614(77)90060-X
Montgomery, R.L., Melaugh, R.A., Lau, C.-C., Meier, G.H., Chan, H.H., Rossini, F.D.: Determination of the energy equivalent of a water solution calorimeter with a standard substance. J. Chem. Thermodyn. 9, 915–936 (1977). doi:10.1016/0021-9614(77)90214-2
Pitzer, K.S.: In: Pitzer, K.S. (ed.) Activity Coefficients in Electrolyte Solutions, Chap. 3. CRC, Boca Raton (1991), revised edn.
Marcus, Y.: Ion Solvation, p. 108. Wiley, Chichester (1985)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guan, W., Xue, WF., Tong, J. et al. The Enthalpy of Solution of the Alanine-Based Ionic Liquid [C4mim][Ala]. J Solution Chem 38, 1463–1469 (2009). https://doi.org/10.1007/s10953-009-9458-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-009-9458-4