Abstract
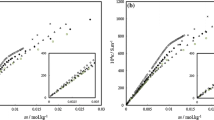
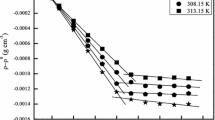
Specific heats of aqueous solutions of lithium perfluoroalkanoates, from C6 to C9, were determined at 298.15 K at concentrations below and above the critical micelle concentration. Infinite dilution apparent molar heat capacities are compared with literature data for corresponding salts with different counterions. Heat capacities of micellization of these surfactants in water were calculated from the specific heat data and also by measurements of the heat of micellization at two temperatures, 298.15 K and 308.15 K. The data were treated under the assumption of the pseudo-phase separation model. The two series of data agree in the case of perfluorononanoate but diverge for perfluorosurfactants with shorter hydrophobic chains. The results are interpreted in terms of the extent of the applicability of the adopted chemical model. Heat capacities of the micellization process obtained from experimental specific heats compare well with literature values relative to the sodium salts of the examined anions.
Similar content being viewed by others
References
Kissa, E. (ed.): Fluorinated Surfactants and Repellents, 2nd edn. Surfactant Science Series, vol. 97. Marcel Dekker Inc., New York (2001)
Muller, N., Simsohn, H.: Investigation of micelle structure by fluorine magnetic resonance. J. Phys. Chem. 75, 942–945 (1971)
Iijima, H., Kato, T., Söderman, O.: Variation in degree of counterion binding to cesium perfluorooctanoate micelles with surfactant concentration studied by 133Cs and 19F NMR. Langmuir 16, 318–323 (2000)
Amato, M.E., Caponetti, E., Chillura Martino, D., Pedone, L.: 1H and 19F NMR investigation on mixed hydrocarbon-fluorocarbon micelles. J. Phys. Chem. B 107, 10048–10056 (2003)
Iijima, H., Koyama, S., Fujio, K., Uzu, Y.: NMR study of the transformation of perfluorinated surfactant solutions. Bull. Chem. Soc. Jpn. 72, 171–177 (1999)
Guo, W., Brown, A., Fung, B.M.: Micelles and aggregates of fluorinated surfactants. J. Phys. Chem. 95, 1829–1836 (1991)
Ristori, S., Martini, G.: EPR lineshape analysis of small and large probes introduced into micellar aqueous solutions of ammonium pentadecafluorooctanoate. Langmuir 8, 1937–1942 (1992)
Mukerjee, P., Gumkowski, M.J., Chan, C.C., Sharma, R.: Determination of critical micellization concentrations of perfluorocarboxylates using ultraviolet spectroscopy: some unusual counterion effects. J. Phys. Chem. 94, 8832–8835 (1990)
Asakawa, T., Miyagishi, S.: Demicellization of sodium perfluorooctanoate and dodecyl sulphate mixtures revealed by pyrene fluorescence quenching. Langmuir 15, 3464–3468 (1999)
Nakano, T.-Y., Sugihara, G., Nakashima, T., Yu, S.-C.: Thermodynamic study of mixed hydrocarbon/fluorocarbon surfactant system by conductometric and fluorimetric techniques. Lamgmuir 18, 8777–8785 (2002)
López-Fontán, J.L., Sarmiento, F., Schulz, P.C.: The aggregation of sodium perfluorooctanoate in water. Colloid Polym. Sci. 283, 862–871 (2005)
Iijima, H., Kato, T., Yoshida, H., Imai, M.: Small-angle X-ray and neutron scattering from dilute solutions of cesium perfluorooctanoate. Micellar growth along two dimensions. J. Phys. Chem. 102, 990–995 (1998)
Berr, S., Jones, R.R.M.: Small-angle neutron scattering from aqueous solutions of sodium perfluorooctanoate above the critical micelle concentration. J. Phys. Chem. 93, 2555–2558 (1989)
Iampietro, D.J., Kaler, E.W.: Phase behavior and microstructure of aqueous mixtures of cetyltrimethtlammonium bromide and sodium perfluorohexanoate. Langmuir 15, 8590–8601 (1999)
Méndez Sierra, J.A., Jańczuk, B., González-Martín, M.L., Bruque, J.M.: Electrical conductivity measurements for the systems decylammonium chloride/water and cesium perfluorooctanoate/water in the isotropic phase. Colloids Surf. A, Physicochem. Eng. Asp. 117, 143–149 (1996)
Hoffmann, H., Platz, G., Rehage, H., Reizlein, K., Ulbricht, W.: Messungen zum aggregationsverhalten perfluorierter alkansäuren. Makromol. Chem. 182, 451–481 (1981)
Muzzalupo, R., Ranieri, G.A., La Mesa, C.: Solution properties of alkali metal perfluoroalkanoates. Colloids Surf. A, Physicochem. Eng. Asp. 104, 327–336 (1995)
La Mesa, C., Sesta, B.: Micelles in perfluorinated surfactant solutions. J. Phys. Chem. 91, 1450–1454 (1987)
Mukerjee, P., Korematsu, K., Okawauchi, M., Sugihara, G.: Effect of temperature on the electrical conductivity and the thermodynamics of micelle formation of sodium perfluorooctanoate. J. Phys. Chem. 89, 5308–5312 (1985)
Gonzalez-Perez, A., Ruso, J.M., Romero, M.J., Blanco, E., Prieto, G., Sarmiento, F.: Application of thermodynamic models to study micellar properties of sodium perfluoroalkyl carboxylates in aqueous solution. Chem. Phys. 313, 245–259 (2005)
La Mesa, C.: Counterion binding to micelles. Ann. Chim. 77, 93–101 (1987)
Gianni, P., Barghini, A., Bernazzani, L., Mollica, V.: Calorimetric investigation of the interaction between lithium perfluorononanoate and poly(ethylene glycol) oligomers in water. Langmuir 22, 8001–8009 (2006)
Johnson, I., Olofsson, G.: Thermodynamics of micelle formation of alkali-metal perfluorononanoates in water. J. Chem. Soc., Faraday Trans. I 84, 551–560 (1988)
Tomasic, V., Chittofrati, A., Kallay, N.: Thermodynamic properties of aqueous solutions of perfluorinated ionic surfactants. Colloids Surf. A, Physicochem. Eng. Asp. 104, 95–99 (1995)
De Lisi, R., Milioto, S., De Giacomo, A., Inglese, A.: Thermodynamic properties of sodium n-perfluoroalkanoates in water and in water + cyclodextrins mixtures. Langmuir 15, 5014–5022 (1999)
González-Martín, M.L., Jańczuk, B., MéndezSierra, J.A., Bruque, J.M.: Volumetric properties of the decylammonium chloride and cesium perfluorooctanoate from density measurements. Colloids Surf. A, Physicochem. Eng. Asp. 148, 213–221 (1999)
Kato, S., Harada, S., Nakashima, H., Nomura, H.: Ultrasonic relaxation and volumetric studies of micelle-monomer exchange process in aqueous solutions of sodium and cesium perfluorooctanoates. J. Colloid Interface Sci. 150, 305–313 (1992)
Perron, G., Desnoyers, J.E.: Volumes and heat capacities of sodium perfluoroalkanoates in water. J. Chem. Eng. Data 42, 172–178 (1997)
González-Perez, A., Ruso, J.M., Prieto, G., Sarmiento, F.: The self-aggregation of sodium perfluorooctanoate in aqueous solution at different temperatures. J. Surfactants Deterg. 7, 387–395 (2004)
Kunieda, H., Shinoda, K.: Krafft points, critical micelle concentrations, surface tension, and solubilising power of aqueous solutions of fluorinated surfactants. J. Phys. Chem. 80, 2468–2470 (1976)
Ikawa, Y., Tsuru, S., Murata, Y., Ōkawauchi, M., Shigematsu, M., Sugihara, G.: A pressure and temperature study on solubility and micelle formation of sodium perfluorodecanoate in aqueous solution. J. Solution Chem. 17, 125–137 (1988)
Gianni, P., Bernazzani, L., Carosi, R., Mollica, V.: Micellization of lithium perfluoroheptanoate and its aggregation on poly(ethylene glycol) oligomers in water. Langmuir 23, 8752–8759 (2007)
Gianni, P., Bernazzani, L., Guido, C.A., Mollica, V.: Calorimetric investigation of the aggregation of lithium perfluorooctanoate on poly(ethylene glycol) oligomers in water. Thermochim. Acta 451, 73–79 (2006)
Gianni, P., Barghini, A., Bernazzani, L., Mollica, V., Pizzolla, P.: Aggregation of cesium perfluorooctanoate on poly(ethylene glycol) oligomers in water. J. Phys. Chem. B 110, 9112–9121 (2006)
Woolley, E.M., Burchfield, T.E.: Model for thermodynamics of ionic surfactant solutions. 2. Enthalpies, heat capacities, and volumes. J. Phys. Chem. 88, 2155–2163 (1984)
Bach, J., Blandamer, M.J., Burgess, J., Cullis, P.M., Soldi, L.G., Bijma, K., Engberts, J.B.F.N., Kooreman, P.A., Kacperska, A., Chowdoji Rao, K., Subha, M.C.: Titration calorimetric and spectrophotometric studies of micelle formation by alkyltrimethylammonium bromide in aqueous solution. J. Chem. Soc., Faraday Trans. 91, 1229–1235 (1995)
Shinoda, K., Hutchinson, E.: Pseudo-phase separation model for thermodynamic calculations on micellari solutions. J. Phys. Chem. 66, 577–582 (1962)
Conti, G., Gianni, P., Papini, A., Matteoli, E.: Apparent molar heat capacity and relative enthalpy of aqueous NaOH between 323 and 523 K. J. Solution Chem. 17, 481–496 (1988)
Conti, G., Gianni, P., Matteoli, E.: Excess enthalpies and excess heat capacities of the ternary system ethanol + tetrahydrofuran + cyclohexane at 298.15 K. Thermochim. Acta 247, 293–313 (1994)
Olofsson, I.V.: Apparent molar heat capacities and volumes of aqueous NaCl, KCl, and KNO3 at 298.15 K. Comparison of Picker flow calorimeter with other calorimeters. J. Chem. Thermodyn. 11, 1005–1014 (1979)
Parker, V.P.: Thermal properties of aqueous uni-univalent electrolytes. U.S. Dept. of Commerce, NSRDS-NBS 2 (1965)
Blanco, E., Messina, P., Ruso, J.M., Prieto, G., Sarmiento, F.: Counterion effect on the solution and thermodynamic properties of lithium perfluoroalkanoates. Mol. Phys. 103, 3271–3281 (2005)
Blanco, E., González-Perez, A., Ruso, J.M., Pedrido, R., Prieto, G., Sarmiento, F.: A comparative study of the physicochemical properties of perfluorinated and hydrogenated amphiphiles. J. Colloid Interface Sci. 288, 247–260 (2005)
De Lisi, R., Milioto, S., Muratore, N.: Thermodynamic evidence of cyclodextrin-micelle interactions. J. Phys. Chem. B 106, 8944–8953 (2002)
Ropers, M.H., Czichocki, G., Brezesinski, G.: Counterion effect on the thermodynamics of micellization of alkyl sulfates. J. Phys. Chem. B 107, 5281–5288 (2003)
Li, Y., Reeve, J., Wang, Y., Thomas, R.K., Wang, J., Yan, H.: Microcalorimetric study on micellization of non-ionic surfactants with a benzene ring or adamantine in their hydrophobic chains. J. Phys. Chem. B 109, 16070–16074 (2005)
Thongngam, M., McClements, D.J.: Influence of pH, ionic strength, and temperature on self-association and interactions of sodium dodecyl sulphate in the absence and presence of chitosan. Langmuir 21, 79–86 (2005)
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Bernazzani, L., Carosi, R., Gianni, P. et al. Heat Capacity of Micellization of Lithium Perfluoroalkanoates in Aqueous Solution. J Solution Chem 38, 1369–1379 (2009). https://doi.org/10.1007/s10953-009-9453-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-009-9453-9