Abstract
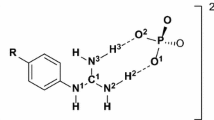
Adduct formation in binary systems of O-phospho-L-serine (Ser-P) with adenosine 5′-monophosphate (AMP), adenosine 5′-diphosphate (ADP) and adenosine 5′-triphosphate (ATP), has been investigated. This study was performed in aqueous solutions using a potentiometric method with computer analysis of the data, together with 13C and 31P NMR spectroscopic measurements. The overall stability constants of the adducts and the equilibrium constants for their formation have been determined. Ion-dipole and ion-ion interactions have been established to occur in the identified noncovalent complexes. An analysis of the equilibrium constants of the reaction has allowed the determination of the effectiveness of the phosphate groups and donor atoms of heterocyclic rings for molecular complex formation. The potential reaction centers are the atoms N(1) and N(7) from the purine base, the phosphate group of the nucleotides, and the phosphate, carboxyl and amine groups from phosphorylated serine. Sites for the interactions in the bioligands have been found on the basis of an equilibrium constant study and an analysis of the changes in the signal positions of their NMR spectra.
Similar content being viewed by others
References
Cohen, P.: The origins of protein phosphorylation. Nat. Cell Biol. 4, E127–E130 (2002). doi:10.1038/ncb0502-e127
Elliot, W.H., Elliot, D.C.: Biochemistry and Molecular Biology, 2nd edn. Oxford University Press, Oxford (2002), p. 296
Yarligana, S., Fuzery, A.K., Ogretir, C., Csizmadia, I.G.: Deciphering the ‘biological Morse-code’: a preliminary ab initio study of phosphoserine. J. Mol. Struct.-Theochem. 666–667, 269–271 (2003). doi:10.1016/j.theochem.2003.08.116
Stryer, L.: Biochemistry. Freeman, New York (1995)
Martin, R.B.: Metal ions binding to nucleoside and nucleotides. In: Xavier, A.V. (ed.) Frontiers in Bioinorganic Chemistry. VCH, Weinheim (1986)
Marzilli, L.G.: Metal complex of nucleic acid derivatives and nucleotides: binding sites and structures. In: Eichhorn, G.L., Marzilli, L.G. (eds.) Metal Ions in Genetic Information Transfer, pp. 47–52. Elsevier/North-Holland, Amsterdam (1981)
Sigel, H.: Complexes of metal ions with various nucleic acids components. In: Berthon, G. (ed.) Handbook of Metal–Ligand Interactions in Biological Fluids, pp. 451–459. Marcel Dekker, New York (1995)
Martin, R.B.: Nucleoside sites for transition metal ion binding. Acc. Chem. Res. 18, 32–38 (1985). doi:10.1021/ar00110a001
De Castro, B., Pereira, J., Gameiro, P., Lima, J.L.: Multinuclear NMR and potentiometric studies on the interaction of zinc and cadmium with cytidine and glycylglycine. The effect of the anion. J. Inorg. Biochem. 45, 53–64 (1992). doi:10.1016/0162-0134(92)84041-K
Lomozik, L.: Metal Complexes with Polyamines. In: Berthon, G. (ed.) Handbook of Metal-Ligand Interactions in Biological Fluids, vol. I, p. 686. Marcel Dekker, New York (1995)
Gasowska, A., Lomozik, L.: Spectroscopic and potentiometric investigation of the solution structure and stability of Ni(II) and Co(II) complexes with adenosine 5′-monophosphate and 1,12-diamino-4,9-diazadodecane (spermine) or 1,11-diamino-4,8-diazaundecane. Polyhedron 21, 745–751 (2002). doi:10.1016/S0277-5387(02)00849-5
Irving, M.H., Miles, M.G., Pettit, L.D.: A study of some problems in determining the stoichiometric proton dissociation constants of complexes by potentiometric titrations using a glass electrode. Anal. Chim. Acta 38, 475–488 (1967). doi:10.1016/S0003-2670(01)80616-4
Gans, P., Sabatini, A., Vacca, A.: Investigation of equilibria in solution. Determination of equilibrium constants with the HYPERQUAD suite of programs. Talanta 43, 1739–1753 (1996). doi:10.1016/0039-9140(96)01958-3
Ingri, N., Kakolowicz, W., Sillen, L.G., Warqvist, B.: High-speed computers as a supplement to graphical methods, V: haltafall, a general program for calculating the composition of equilibrium mixtures. Talanta 14, 1261–1286 (1967). doi:10.1016/0039-9140(67)80203-0
Lomozik, L., Jaskolski, M., Wojciechowska, A.: A multistage verification procedure for the selection of models in the studies of complex formation equilibria. Pol. J. Chem. 65, 1797–1807 (1991)
Glasoe, P.K., Long, F.A.: Use of glass electrodes to measure acidities in deuterium oxide. J. Phys. Chem. 64, 188–189 (1960). doi:10.1021/j100830a521
Lomozik, L., Gasowska, A.: Complexes of copper(II) with spermine and non-covalent interactions in the systems including nucleosides or nucleotides. J. Inorg. Biochem. 72, 37–47 (1998). doi:10.1016/S0162-0134(98)10060-0
Lomozik, L., Gasowska, A., Bregier, R.: Coordination mode of adenosine 5′-diphosphate in ternary systems containing Cu(II), Cd(II) or Hg(II) ions and polyamines. J. Inorg. Biochem. 98, 1319–1330 (2004). doi:10.1016/j.jinorgbio.2004.04.007
Gasowska, A., Lomozik, L.: Spectroscopic and potentiometric investigation of the solution structure and stability of Ni(II) and Co(II) complexes with adenosine 5′-monophosphate and 1,12-diamino-4,9-diazadodecane (spermine) or 1,11-diamino-4,8-diazaundecane. Polyhedron 21, 745–751 (2002). doi:10.1016/S0277-5387(02)00849-5
Folsch, G., Osterberg, R.: The apparent acid ionization constants of some phosphorylated peptides and related compounds. J. Biol. Chem. 234, 2298–2303 (1959)
Martell, A.E., Smith, R.M.: Critical Stability Constants. Plenum Press, New York (1974)
Khalil, M.J.: Solution equilibria and stabilities of binary and ternary complexes with N-(2-acetamido) iminodiacetic acid and ribonucleotides (AMP, ADP, and ATP). J. Chem. Eng. Data 45, 837–840 (2000). doi:10.1021/je000041a
Zachariou, M., Traverso, I., Spiccia, L., Hearn, M.T.W.: Potentiometric investigations into the acid-base and metal ion binding properties of immobilized metal ion affinity chromatographic (IMAC) adsorbents. J. Phys. Chem. 100, 12680–12690 (1996). doi:10.1021/jp9601476
Bianchi, E.M., Ali, S., Sajadi, A., Song, B., Sigel, H.: Stabilities and isomeric equilibria in aqueous solution of monomeric metal ion complexes of adenosine 5′-diphosphate (ADP3−) in comparison with those of adenosine 5-monophosphate (AMP2−). Chem. Eur. J. 9, 881–892 (2003). doi:10.1002/chem.200390109
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jastrzab, R., Lomozik, L. Effectiveness of Phosphate Groups in Noncovalent Interactions in Binary Adenosine Nucleotides/Phosphoserine Aqueous Systems. J Solution Chem 38, 35–46 (2009). https://doi.org/10.1007/s10953-008-9352-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-008-9352-5