Abstract
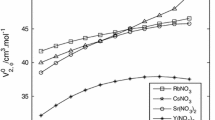
The viscosities of aqueous solutions of lithium, sodium, potassium, rubidium and caesium cyclohexylsulfamates were measured at 293.15, 298.15, 303.15, 313.15 and 323.15 K. The relative viscosity data were analyzed and interpreted in terms of the Kaminsky equation, η r=1+Ac 1/2+Bc+Dc 2. The viscosity A-coefficient was calculated from the Falkenhagen-Dole theory. The viscosity B-coefficients are positive and relatively large. Their temperature coefficient ∂ B/∂ T is negative or near zero for lithium and sodium salts whereas for potassium, rubidium and caesium salts it is positive. The viscosity D-coefficient is positive. This was explained by the size of the ions, structural solute–solute interactions, hydrodynamic effect, and by higher terms of the long-range Debye-Hückel type of forces. From the viscosity B-coefficients the thermodynamic functions of activation of viscous flow were calculated. The limiting partial molar Gibbs energy of activation of viscous flow of the solute was divided into contributions due to solvent molecules and the solute in the transition state. The activation energy of the solvent molecules was calculated using the limiting Gibbs energy of activation for the conductance of the solute ions. The activation energy of the solvent molecules was then discussed in terms of the nature of the alkali-metal ions and their influence on the structure of water. The limiting activation entropy and enthalpy of the solute for activation of viscous flow were interpreted by ion-solvent bond formation or breaking in the transition state of the solvent. The hydration numbers of the investigated electrolytes were calculated from the specific viscosity of the solutions.
Similar content being viewed by others
References
O’Brien-Nabors, L., Gelardi, R.C.: Alternative sweeteners: an overview. In: Alternative Sweeteners, 2nd edn. Marcel Dekker, New York (1991)
Mathlouthi, M., Bressan, C., Portmann, M.O., Serghat, S.: Role of water. In: Mathlouthi, M., Kanters, J.A., Birch, G.G. (eds.) Sweet Taste Chemoreception, pp. 141–172. Elsevier, London & New York (1993)
Jones, G., Dole, M.: The viscosity of aqueous solutions of strong electrolytes with special reference to barium chloride. J. Am. Chem. Soc. 51, 2950–2964 (1929)
Jenkins, H.D.B., Marcus, Y.: Viscosity B-coefficients of ions in solution. Chem. Rev. 95, 2695–2724 (1995)
Feakins, D., Waghorne, W.E., Lawrence, K.G.: The viscosity and structure of solutions. A new theory of the Jones-Dole B-coefficient and the related activation parameters – application to aqueous-solutions. J. Chem. Soc. Faraday Trans. 82(1), 563–568 (1986)
Feakins, D., Bates, F.M., Waghorne, W.E., Lawrence, K.G.: Relative viscosities and quasi-thermodynamics of solutions of tert-butyl alcohol in the methanol-water system – a different view of the alkyl water interaction. J. Chem. Soc. Faraday Trans. 89, 3381–3388 (1993)
Feakins, D., Canning, F.M., Mullally, J.J., Waghorne, W.E.: The thermodynamics of solutions. Pure Appl. Chem. 61, 133–142 (1989)
Rudan-Tasic, D., Klofutar, C., Horvat, J.: Viscosity of aqueous solutions of some alkali cyclohexylsulfamates at 25.0 °C. Food Chem. 86, 161–167 (2004)
Cannon, M.R., Manning, R.E., Bell, J.D.: Viscosity measurement. The kinetic energy correction and a new viscometer. Anal. Chem. 32, 355–358 (1960)
Riddick, J.A., Bunger, W.B., Sakano, T.K.: Organic Solvents, Physical Properties and Methods of Purification, 4th edn. Wiley, New York (1986)
Kaminsky, M.: The concentration and temperature dependence of the viscosity of aqueous solutions of strong electrolytes. III. KCl, K2SO4, MgCl2, BeSO4, and MgSO4 solutions. Z. Phys. Chem. 12, 206–231 (1957)
Klofutar, C., Horvat, J., Rudan-Tasic, D.: Apparent molar volume and apparent molar expansibility of rubidium, caesium, and ammonium cyclohexylsulfamate in aqueous solution. Monatsh. Chem. 137, 1151–1162 (2006)
Klofutar, C., Rudan-Tasic, D.: Apparent molar volume and apparent molar expansibility of lithium, sodium, potassium, and tetramethylammonium cyclohexylsulfamate in aqueous solution. Monatsh. Chem. 136, 1727–1736 (2005)
Falkenhagen, H., Dole, M.: Viscosity of electrolyte solutions and its significance to the Debye theory. Physik. Z. 30, 611–622 (1929)
Archer, D.G., Wang, P.M.: The dielectric-constant of water and Debye-Hückel limiting law slopes. J. Phys. Chem. Ref. Data 19, 371–411 (1990)
Harned, R.S., Owen, B.B.: The Physical Chemistry of Electrolytic Solutions, 3rd edn. Reinhold, New York (1958)
Rudan-Tasič, D., Župec, T., Klofutar, C., Bešter-Rogač, M.: A conductometric study of aqueous solutions of some cyclohexylsulfamates. J. Solution Chem. 34, 631–644 (2005)
Rudan-Tasič, D., Klofutar, C., Bešter-Rogač, M.: The electric conductivities of aqueous solutions of rubidium and cesium cyclohexylsulfamates, potassium acesulfame and sodium saccharin. Acta Chim. Slov. 53, 324–330 (2006)
Klofutar, C., Luci, M., Abramović, H.: The thermodynamics of dissociation of cyclohexylsulfamic acid in aqueous solution. Physiol. Chem. Phys. Med. NMR 31, 1–8 (1999)
Out, D.J.P., Los, J.M.: Viscosity of aqueous-solutions of univalent electrolytes from 5 to 95 °C. J. Solution Chem. 9, 19–35 (1980)
Falkenhagen, H.: Bemerkung zur inneren Reibung starker Electrolyte in sehr verdünnte Lösungen. Physik. Z. 32, 365–369 (1931)
Falkenhagen, H.: The quantitative limiting law for the viscosity of strong binary electrolytes. Physik. Z. 32, 745–764 (1931)
Kaminsky, M.: Investigation of the interaction between ion and solvent by measurement of viscosity. Z. Naturforsch. 12a, 424–433 (1957)
Lencka, M.M., Anderko, A., Sanders, S.J., Young, R.D.: Modeling viscosity of multicomponent electrolyte solutions. Int. J. Thermophys. 19, 367–378 (1998)
Desnoyers, J.E., Perron, G.: The viscosity of aqueous solutions of alkali and tetraalkylammonium halides at 25 °C. J. Solution Chem. 1, 199–212 (1972)
Glasstone, S., Laidler, K.J., Eyring, H.: The Theory of Rate Processes. McGraw-Hill, New York (1941)
Feakins, D., Freemantle, D.J., Lawrence, K.G.: Transition-state treatment of relative viscosity of electrolyte solutions – applications to aqueous, non-aqueous and methanol+water systems. J. Chem. Soc. Faraday Trans. 70, 795–806 (1974)
Waghorne, W.E.: Viscosities of electrolyte solutions. Phil. Trans. R. Soc. London Ser. A 359, 1529–1543 (2001)
Brummer, S.B., Hills, G.J.: Kinetics of ionic conductance. Part 1 – Energies of activation and the constant volume principle. J. Chem. Soc. Faraday Trans. 5, 1816–1822 (1961)
Hickey, K., Waghorne, W.E., Sacco, A.: Comparison of the activation free energies for viscous flow and for diffusion in dilute solutions, derivation of the expression for the effect of the solute on the activation free energy of diffusion of the solvent, and application to solutions of N,N-dimethylformamide in water, methanol, and acetonitrile. J. Phys. Chem. A 105, 1093–1096 (2001)
Einstein, A.: A new determination of molecular dimensions. Ann. Physik 19, 289–306 (1911)
Einstein, A.: Berichtung zu meiner Arbeit, eine neue Bestimmung der molekul Dimensionen. Ann. Physik 34, 591–592 (1911)
Linow, K.J.: Philipp, B.: The concentration dependency of viscosity – a new method for the discovery of solutes. Z. Phys. Chem. 265, 321–329 (1984)
Neilson, G.W., Mason, P.E., Ramos, S., Sullivan, D.: Neutron and X-ray scattering studies of hydration in aqueous solutions. Phil. Trans. R. Soc. Lond. Ser. A 359, 1575–1591 (2001)
Lee, S.H., Rasaiah, J.C.: Molecular dynamics simulation of ion mobility. 2. Alkali metal and halide ions using the SPC/E model for water at 25 °C. J. Phys. Chem. 100, 1420–1425 (1996)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Horvat, J., Bešter-Rogač, M., Klofutar, C. et al. Viscosity of Aqueous Solutions of Lithium, Sodium, Potassium, Rubidium and Caesium Cyclohexylsulfamates from 293.15 to 323.15 K. J Solution Chem 37, 1329–1342 (2008). https://doi.org/10.1007/s10953-008-9311-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-008-9311-1