Abstract
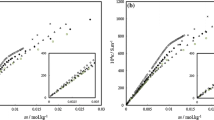
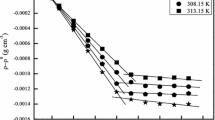
The power-time curves for the micelle formation process were determined for two anionic surfactants, sodium laurate (SLA) and sodium dodecyl sulfate (SDS), in mixed alcohol + N,N-dimethylacetamide (DMA) solvent using titration microcalorimetry. From the data of the lowest point and the area of the power-time curves, their critical micelle concentration (CMC) and ΔH om were obtained. The other thermodynamic functions of the micellization process (ΔG om and ΔS om ) were also calculated with thermodynamic equations. For both surfactants, the effects of the carbon number (chain length) of the alcohol, the concentration of alcohol, and the temperature on the CMC and thermodynamic functions are discussed. For systems containing identical concentrations of a different alcohol, values of the CMC, ΔH om and ΔS om increased whereas ΔG om decreased with increasing temperature. For systems containing an identical alcohol concentration at the same temperature, values of the CMC, ΔH om ,ΔG om and ΔS om decrease with increasing carbon number of alcohol. For systems containing the same alcohol at the same temperature, the CMC and ΔG om values increase whereas ΔH om and ΔS om decrease with increasing alcohol concentration.
Similar content being viewed by others
References
Evans, D.F., Miller, D.D.: Organized Solutions. Dekker, New York (1992)
Nakano, T., Sugihara, G., Nakashima, T., Yu, S.: Thermodynamic study of mixed hydrocarbon/fluorocarbon surfactant system by conductometric and fluorimetric techniques. Langmuir 18, 8777–8785 (2002)
Wang, X., Li, Y., Wang, J., Wang, Y., Ye, J., Yan, H.: Interaction of cationic gemini surfactants with hydrophobically modified poly(acrylamides) studied by fluorescence and microcalorimetry. J. Phys. Chem. B 109, 12850–12855 (2005)
Li, Y., Reeve, J., Wang, Y., Thomas, R.K., Wang, J., Yan, H.: Microcalorimetric study on micellization of nonionic surfactants with a benzene ring or adamntane in their hydrophobic chains. J. Phys. Chem. B 109, 16070–16074 (2005)
Akhter, M.S.: Effect of solubilization of alcohols on critical micelle concentration of non-aqueous micelle solution. Colloids Surf. A Physicochem. Eng. Asp. 157, 203–210 (1999)
Akhter, M.S., Sadeq, M., Lawi, A.: Influence of alcohols on the critical micelle concentration of non-aqueous micelle solution. Colloids Surf. A Physicochem. Eng. Asp. 164, 247–255 (2000)
Salim, M., Akhter, M.S., Sadeq, M., Lawi, A.: The effect of organic additives on critical micelle concentration of non-aqueous micelle solution. Colloids Surf. A Physicochem. Eng. Asp. 175, 311–320 (2000)
Liu, H., Lin, R., Zhang, H.: Enthalpic titrations of amino acids with glucose aqueous solutions at 298.15 K. J. Solution Chem. 32, 977–985 (2003)
Liu, H., Lin, R., Zhang, H.: Enthalpies of dilution and enthalpies of mixing of amino-acids with sucrose in aqueous solution at 298.15 K. J. Solution Chem. 35, 679–688 (2006)
Yu, X.-F., Wu, L.-L., Zhang, H.-L.: Microcalorimetric study on the formation of reversed micelle in P204Li organic phase. Chin. J. Appl. Chem. 3, 263–266 (2000)
Chauhan, M.S., Kumar, G., Kumar, A., Chauhan, S.: Micellization of ionic surfactants in aqueous-rich region of organic solvants: A conductometric study of micellization behaviour of sodium dodecylsulfate in aqueous-rich region of 1-BuOH, 2-BuOH, t-BuOH at different temperatures. Colloids Surf. 166, 51–57 (2000)
Hierrezuelo, J.M., Aguiar, J., Carnero Ruiz, C.: Interactions in binary mixed systems involving a sugar-based surfactant and different n-alkyltrimethylammonium bromides. J. Colloid Interface Sci. 294, 449–457 (2006)
Rosen, M.J.: Surfactants and Interfacial Phenomena. Wiley, New York (1978)
Li, G.-Z., Rong, G.: Theory of Latex Emulsion and Application. Petroleum Industry Press, Beijing (1995)
Attwood, D., Florence, A.T.: Surfactant Systems—Their Chemistry, Pharmacy and Biology. Chapman and Hall, London (1983)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, HL., Kong, Z., Yan, YM. et al. Study of an Alcohol’s Influence on the CMC and Thermodynamic Functions of Anionic Surfactants in DMA/Long-chain Alcohol Solutions Using a Microcalorimetric Method. J Solution Chem 37, 1631–1644 (2008). https://doi.org/10.1007/s10953-008-9290-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-008-9290-2