Abstract
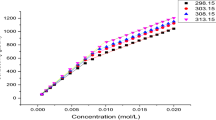
The complexes of lysozyme with the anionic surfactant sodium dodecyl sulfate (SDS) and the cationic surfactant dodecyltrimethylammonium bromide (DTAB) have been investigated by isothermal titration calorimetry at pH=7.0 and 27 °C in a phosphate buffer. A new direct calorimetric method was applied to follow the protein denaturation and study the effect of surfactants on the stability of proteins. The extended solvation model was used to represent the enthalpies of lysozyme + SDS interaction over the whole range of SDS concentrations. The solvation parameters recovered from the new equation are attributed to the structural change of lysozyme and its biological activity. At low SDS concentrations, the binding is mainly electrostatic with some simultaneous interaction of the hydrophobic tail with nearby hydrophobic regions of lysozyme. These initial interactions presumably cause some protein unfolding and expose additional hydrophobic sites. The induced enthalpy of denaturation of lysozyme by SDS is 160.81±0.02 kJ⋅mol−1. The lysozyme-DTAB complexes behave very differently from those of the lysozyme-SDS complexes. SDS induces a stronger unfolding of lysozyme than DTAB. The induced enthalpy of lysozyme denaturation by DTAB is 86.46±0.02 kJ⋅mol−1.
Similar content being viewed by others
References
Lapanje, S.: Physicochemical Aspect of Protein Denaturation. Wiley, New York (1978)
Saboury, A.A., Moosavi-Movahedi, A.A.: Derivation of the thermodynamic parameters involved in the elucidation of protein thermal profiles. Biochem. Educ. 23, 164–167 (1995)
Saboury, A.A., Shamsaei, A.A., Moosavi-Movahedi, A.A., Mansori-Torshizi, H.: Thermodynamics of binding 2,2′-bipyridineglycinato palladium(II) chloride on human serum albumin. J. Chin. Chem. Soc. 46, 917–922 (1999)
Saboury, A.A., Karbassi, F.: Thermodynamic studies on the interaction of calcium with alpha-amylase. Thermochim. Acta 362, 121–129 (2000)
Saboury, A.A., Atri, M.S., Sanati, M.H., Moosavi-Movahedi, A.A., Hakimelahi, G.H., Sadeghi, M.: A thermodynamic study on the interaction between magnesium ion and human growth hormone. Biopolymers 81, 120–126 (2006)
Bordbar, A.K., Saboury, A.A., Moosavi-Movahedi, A.A.: The shapes of Scatchard plots for systems with two sets of binding sites. Biochem. Educ. 24, 172–175 (1996)
Saboury, A.A., Bordbar, A.K., Moosavi-Movahedi, A.A.: The enthalpy of unfolding for jack bean urease with interaction of n-alkyl trimethylammonium bromides. J. Chem. Thermodyn. 28, 1077–1082 (1996)
Bordbar, A.K., Moosavi-Movahedi, A.A., Saboury, A.A.: Comparative thermodynamical stability of bovine and pigeon hemoglobin by interaction with sodium n-dodecyl sulphate. Thermochim. Acta 287, 343–349 (1996)
Saboury, A.A., Bordbar, A.K., Moosavi-Movahedi, A.A.: Resolution of two sets of binding sites for cationic surfactant-urease interaction. Bull. Chem. Soc. Jpn. 69, 3031–3035 (1996)
Bordbar, A.K., Saboury, A.A., Housaindokht, M.R., Moosavi-Movahedi, A.A.: Statistical effects of the binding of ionic surfactant to protein. J. Colloid Interfacial Sci. 192, 415–419 (1997)
Moosavi-Movahedi, A.A., Nazari, K., Saboury, A.A.: Thermodynamic denaturation of horseradish peroxidase with sodium n-dodecyl sulphate and n-dodecyl trimethylammonium bromide. Colloid Surf. B Biointerfaces 9, 123–130 (1997)
Nazari, K., Saboury, A.A., Moosavi-Movahedi, A.A.: Enthalpy investigation for elucidation of the transition concentration for the interaction of horseradish peroxidase with surfactants. Thermochim. Acta 302, 131–135 (1997)
Bathaie, S.Z., Moosavi-Movahedi, A.A., Saboury, A.A.: Energetic and binding properties of DNA upon interaction with dodecyl trimethylammonium bromide. Nucleic Acid Res. 27, 1001–1005 (1999)
Moosavi-Movahedi, A.A., Saboury, A.A.: Elucidation of binding sites for protein denaturation by surfactant. J. Chem. Soc. Pak. 21, 248–259 (1999)
Housaindokht, M.R., Chamani, J., Saboury, A.A., Moosavi-Movahedi, A.A.: Three binding sets analysis of α-lactalbumin with interaction of tetradecyl trimethylammonium bromide. Bull. Korean Chem. Soc. 22, 145–148 (2001)
Ajloo, D., Moosavi-Movahedi, A.A., Hakimelahi, G.H., Saboury, A.A., Gharibi, H.: The effect of dodecyl trimethylammonium bromide on the formation of methemoglobins and hemichrome. Colloid Surf. B Biointerfaces 26, 185–196 (2002)
Bathaie, S.Z., Moosavi-Movahedi, A.A., Ranjbar, B., Saboury, A.A.: The mechanistic study of the histone H1-DNA complex dissociation by sodium n-dodecyl sulphate. Colloid Surf. B Biointerfaces 28, 17–25 (2003)
Chamani, J., Moosavi-Movahedi, A.A., Saboury, A.A., Gharanfoli, M., Hakimelahi, G.H.: Calorimetric indication of the molten globule-like state of cytochrome c induced by n-alkyl sulfates at low concentrations. J. Chem. Thermodyn. 35, 199–207 (2003)
Karbassi, F., Haghbeen, K., Saboury, A.A., Ranjbar, B., Moosavi-Movahedi, A.A.: Activity, structural and stability changes of mushroom tyrosinase by sodium dodecyl sulfate. Colloid Surf. B Biointerfaces 32, 137–143 (2003)
Gheibi, N., Saboury, A.A., Haghbeen, K., Moosavi-Movahedi, A.A.: Activity and structural changes of mushroom tyrosinase induced by n-alkyl sulfates. Colloid Surf. B Biointerfaces 45, 104–107 (2005)
Moosavi-Movahedi, A.A.: Thermodynamics of protein denaturation by sodium dodecyl sulfate. J. Iran. Chem. Soc. 2, 189–196 (2005)
Saboury, A.A.: A review on the ligand binding studies by isothermal titration calorimetry. J. Iran. Chem. Soc. 3, 1–21 (2006)
Pirzadeh, P., Moosavi-Movahedi, A.A., Hemmatinejad, B., Ahmad, F., Shamsipur, M.D., Saboury, A.A.: Chemometric studies of lysozyme upon interaction with sodium dodecyl sulfate and β-cyclodextrin. Colloid Surf. B Biointerfaces 52, 31–38 (2006)
Saboury, A.A.: New methods for data analysis of isothermal titration calorimetry. J. Therm. Anal. Calor. 72, 93–103 (2003)
Saboury, A.A.: A simple method for determination of binding isotherm by isothermal titration calorimetry and its application to the interaction between Cu2+ and myelin basic protein. J. Therm. Anal. Calor. 77, 997–1004 (2004)
Saboury, A.A., Atri, M.S., Sanati, M.H., Moosavi-Movahedi, A.A., Haghbeen, K.: Effects of calcium binding on the structure and stability of human growth hormone. Int. J. Biolog. Macromol. 36, 305–309 (2005)
Atri, M.S., Saboury, A.A., Rezaei-Tavirani, M., Sanati, M.H., Moosavi-Movahedi, A.A., Sadeghi, M., Mansuri-Torshizi, H., Khodabandeh, N.: Binding properties and conformational change of human growth hormone upon interaction with Fe+3. Thermochim. Acta 438, 178–183 (2005)
Saboury, A.A., Ghourchaei, H., Sanati, M.H., Atri, M.S., Rezaei-Tawirani, M., Hakimelahi, G.H.: Binding properties and structural changes of human growth hormone upon interaction with cobalt ion. J. Therm. Anal. Calor. 89, 921–927 (2007)
Saboury, A.A.: Application of a new method for data analysis of isothermal titration calorimetry in the interaction between human serum albumin and Ni2+. J. Chem. Thermodyn. 35, 1975–1981 (2003)
Rezaei Behbehani, G.: Application of a new method to reproduce the enthalpies of transfer of NaI from water to aqueous methanol, ethanol and i-PrOH solvent systems at 289.15 K. Bull. Korean Chem. Soc. 26, 238–240 (2005)
Rezaei Behbehani, G.: Application of the new solvation theory to reproduce the enthalpies of transfer of LiBr, tetrabuthylammonium bromide and tetrapenthylamonium bromide from water to aqueous acetonitrile at 298 K. Acta Chim. Slovenica 52, 282–285 (2005)
Rezaei Behbehani, G., Tazikeh, E., Saboury, A.A.: Using the new developed equation to reproduce the enthalpies of transfer of urea from water to aqueous ethanol, propan-1-ol and acetonitrile at 298 K. Bull. Korean Chem. Soc. 27, 208–210 (2006)
Rezaei Behbehani, G., Ghamamy, S.: Enthalpies of transfer of formamide, N-methylformamide and N,N-dimethylformamide from water to aqueous acetonitrile mixtures at 298 K. Thermochim. Acta 444, 71–76 (2006)
Rezaei Behbehani, G., Waghorne, W.E.: Enthalpies of transfer of acetonitrile from water to aqueous methanol, ethanol and dimethylsulphoxide mixtures at 298.15 K. Thermochim. Acta 448, 37–42 (2006)
Rezaei Behbehani, G., Tazikeh, E., Saboury, A.A.: Using the extension coordination model (ECM) to reproduce the enthalpies of transfer of tetraethylurea from water to aqueous ethanol, propan-1-ol and acetonitrile at 298 K. Acta Chim. Slovenica 53, 363–369 (2006)
Rezaei Behbehani, G., Saboury, A.A.: Using a new solvation model for thermodynamic study on the interaction of nickel with human growth hormone. Thermochim. Acta 452, 76–79 (2007)
Rezaei Bebbehani, G., Dunnion, D., Falvey, P., Hickey, K., Meade, M., McCarthy, Y., Symons, M.C.R., Waghorne, W.E.: Nonelectrolyte solvation in aqueous dimethyl sulfoxide—A calorimetric and infrared spectroscopic study. J. Solution Chem. 29, 521–539 (2000)
Rezaei Behbehani, G.: Enthalpies of transfer of tetraalkylammonium bromides and CsBr from water to aqueous DMF at 298.15 K. J. Solution Chem. 8, 939–945 (2007)
Rezaei Behbehani, G., Saboury, A.A.: A thermodynamic study on the binding of magnesium with human growth hormone. J. Therm. Anal. Calor. 89, 859–863 (2007)
Donald, H., Ackers, G.: Calorimetric determination of denaturation enthalpy for lysozyme in guanidine hydrochloride. J. Biol. Chem. 18, 5845–5851 (1971)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rezaei Behbehani, G., Saboury, A.A. & Taleshi, E. A Comparative Study of the Direct Calorimetric Determination of the Denaturation Enthalpy for Lysozyme in Sodium Dodecyl Sulfate and Dodecyltrimethylammonium Bromide Solutions. J Solution Chem 37, 619–629 (2008). https://doi.org/10.1007/s10953-008-9267-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-008-9267-1