Abstract
The complexation behavior of eight M–(buffer) x –(OH) y systems involving two divalent ions (cobalt and nickel) and four zwitterionic biological buffers (AMPSO, DIPSO, TAPS and TAPSO) were characterized. Complex formation was detected for all eight M–(buffer) x –(OH) y systems studied, but fully defined final models were obtained for only four of these systems. For systems involving cobalt or nickel with AMPSO or TAPS, a complete characterization of the systems was not possible in the studied buffer pH-range.
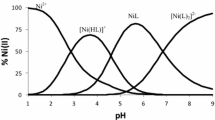
Metal complexation was studied by glass-electrode potentiometry (GEP) and UV-Vis spectroscopy at 25.0 °C and I=0.1 mol⋅dm−3 KNO3 ionic strength. For the Ni–(L) x –(OH) y and Co–(L) x –(OH) y systems, with L = TAPSO or DIPSO, the proposed final models and overall stability constants were obtained by combining results from both techniques. For the Ni–(L) x –(OH) y systems, the measured values of the stability constants are log 10 β NiL=3.0±0.1 and log 10 β NiL2=4.8±0.1 for L = TAPSO, and log 10 β NiL=2.7±0.1 and log 10 β NiL2=4.6±0.1 for L = DIPSO. For the Co–(L) x –(OH) y systems, the overall stability constants are log 10 β CoL=2.2±0.1, log 10 β CoL2=3.6±0.2 and log 10 β CoL(OH)=7.6±0.1 for L = TAPSO, and log 10 β CoL=2.0±0.1 and log 10 β CoL(OH)=7.8±0.1 for L = DIPSO. For both buffers, the CoL(OH) species is characterized by a major structural rearrangement.
Similar content being viewed by others
References
Good, N.E., Winget, G.D., Winter, W., Connolly, T.N., Izawa, S., Singh, R.M.M.: Hydrogen ion buffers for biological research. Biochemistry 5, 467–477 (1966)
Ferguson, W.J., Braunschweiger, K.I., Braunschweiger, W.R., Smith, J.R., McCormick, J.J., Wasmann, C.C., Jarvis, N.P., Bell, D.H., Good, N.E.: Hydrogen ion buffers for biological research. Anal. Biochem. 104, 300–310 (1980)
Martell, A.E., Smith, R.M.: NIST Standard Reference Database 46, Version 8.0, NIST Critically Selected Stability Constants of Metal Complexes Database, U.S. Department of Commerce, National Institute of Standards and Technology (2004)
Nakon, R., Krishnamoorthy, C.R.: Free-metal ion depletion by Good buffers. Science 221, 749–750 (1983)
Pope, J.M., Stevens, P.R., Angotti, M.T., Nakon, R.: Free metal ion depletion by “Good’s” buffers, II: N-(2-acetamido)2-aminoethanesulfonic acid (ACESH): complexes with calcium(II), magnesium(II), manganese(II), cobalt(II), zinc(II), nickel(II) and copper(II). Anal. Biochem. 103, 214–221 (1980)
Flaschks, H.A.: EDTA Titrations. Pergamon, New York (1959)
Machado, C.M.M., Cukrowski, I., Gameiro, P., Soares, H.M.V.M.: Challenges in modeling and optimisation of stability constants in the study of metal complexes with monoprotonated ligands, part I: a glass electrode potentiometric and polarographic study of a Cu-TAPSO-OH system. Anal. Chim. Acta 493, 105–119 (2003)
Azab, H.A., Orabi, A.S., El-Salam, E.T.A.: Role of biologically important zwitterionic buffer secondary ligands on stability of the mixed-ligands complexes of divalent metal ions and adenosine 5′-mono-, 5′-di-, and 5′-triphosphate. J. Chem. Eng. Data 46, 346–354 (2001)
Machado, C.M.M., Scheerlinck, S., Cukrowski, I., Soares, H.M.V.M.: Challenges in modeling and optimisation of stability constants in the study of metal complexes with monoprotonated ligands, part III: a glass electrode potentiometric and polarographic study of Cu-DIPSO-OH system. Anal. Chim. Acta 518, 117–126 (2004)
Machado, C.M.M., Soares, H.M.V.M.: Challenges in modeling and optimization of stability constants in the study of metal complexes with monoprotonated ligands, part IV: a glass electrode potentiometric and polarographic study of Cu-(TAPS) x -(OH) y system. Talanta 71, 1352–1363 (2007)
May, P.M., Murray, K., Williams, D.R.: The use of glass electrodes for the determination of formation constants, II: simulation of titration data. Talanta 32, 483–489 (1985)
May, P.M., Murray, K., Williams, D.R.: The use of glass electrodes for the determination of formation constants, III: optimization of titration data: the ESTA library of computer programs. Talanta 35, 825–830 (1988)
Marsicano, F., Monberg, C., Martincigh, B.S., Murray, K., May, P.M., Williams, D.R.: The existence and stability of mixed-ligand complexes in aqueous solutions containing zinc and cyanide ions at elevated pH values. J. Coord. Chem. 16, 321–339 (1988)
Bugarin, M.G., Filella, M.: The formation constants of dimethylthallium(III)-glutathione complexes in aqueous solution. J. Inorg. Biochem. 73, 17–29 (1999)
Gans, P., Sabatini, A., Vacca, A.: Determination of equilibrium constants from spectrophometric data obtained from solutions of known pH: the program pHab. Ann. Chim. 89, 45–49 (1999)
Nicholls, D.: Complexes and First-Row Transition Elements. Macmillan, London (1974)
Leggett, D.J.: Numerical analysis of multicomponent spectra. Anal. Chem. 49, 276–281 (1977)
Lever, A.B.P.: Inorganic Electronic Spectroscopy. Elsevier, Amsterdam (1968)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Machado, C.M.M., Gameiro, P. & Soares, H.M.V.M. Complexation of M–(buffer) x –(OH) y Systems Involving Divalent Ions (Cobalt or Nickel) and Zwitterionic Biological Buffers (AMPSO, DIPSO, TAPS and TAPSO) in Aqueous Solution. J Solution Chem 37, 603–617 (2008). https://doi.org/10.1007/s10953-008-9265-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-008-9265-3