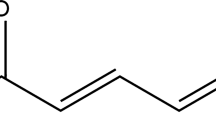
Abstract
A kinetic study of the alkylating potential of the sorbic acid + NaOH and sorbic acid + KOH systems was performed in 7:3 (volume/volume) water + dioxane solvent mixtures. The following conclusions were drawn. First, the sorbic acid + sorbate system shows alkylating activity on the nucleophile 4-(p-nitrobenzyl)pyridine (NBP), which is used as a trap for alkylating agents having nucleophilic characteristics similar to DNA bases. Second, the maximum alkylating capacity is observed in the pH = 5.0 to 6.5 range. Third, the alkylation reactions comply with the rate equation r=k alk[H+][S][NBP]/(K a +[H+]), with K a being the dissociation constant of sorbic acid. Fourth, an enthalpy–entropy (ΔH #/ΔS #) compensation effect for activation quantities is observed by comparing NBP alkylation reactions due to sorbic acid + NaOH, sorbic acid + KOH, as well as potassium sorbate + HCl mixtures. Fifth, the results may help to establish suitable expiration times for products preserved with sorbic acid.
Similar content being viewed by others
References
Thakur, B.R., Singh, K., Arya, S.S.: Chemistry of sorbates—a basic perspective. Food Rev. Int. 10, 71–91 (1994)
Dacosta, Y.: L’acide sorbique et les sorbates. In: CDIUPA, Massy (1994)
U.S. Food and Drug Administration, Center for Food Safety and Applied Nutrition: FDA List of Food Additives that are Generally Recognized as Safe (GRAS). Rockville, MD (2005)
Ferrand, C., Marc, F., Fritsch, P., Cassand, P., de Saint Blanquat, G.: Genotoxicity study of reaction products of sorbic acid. J. Agric. Food Chem. 48, 3605–3610 (2000)
Münzer, R., Guigas, C., Renner, H.W.: Reexamination of potassium sorbate and sodium sorbate for possible genotoxic potential. Food Chem. Toxicol. 28, 397–401 (1990)
Schlater, J., Würgler, F.E., Kränzlin, R., Maier, P., Hollinger, E., Graf, U.: The potential genotoxicity of sorbates: effects on cell cycle in vitro in V79 cells and somatic mutations in Drosophila. Food Chem. Toxicol. 30, 843–851 (1992)
Würgler, F.E., Schlatter, J., Maier, P.: The genotoxicity status of sorbic acid, potassium sorbate and sodium sorbate. Mutat. Res. 283, 107–111 (1992)
Wedzicha, B.L., Brook, M.A.: Reaction of sorbic acid with nucleophiles: preliminary studies. Food Chem. 31, 29–40 (1989)
Khandelwal, G.D., Wedzicha, B.L.: Nucleophilic reactions of sorbic acid. Food Addit. Contam. 7, 685–694 (1990)
Verbiscar, A.J., Campbell, K.N.: Unsaturated six-membered ring lactams. J. Org. Chem. 29, 2472–2474 (1974)
Khandelwal, G.D., Wedzicha, B.L.: Derivatives of sorbic acid-thiol reaction products. Food Chem. 37, 159–169 (1990)
Kito, Y., Namiki, M., Tsuji, K.: A new N-nitropyrrole. 1,4-dinitro-2-methylpyrrole formed by the reaction of sorbic acid with sodium nitrite. Tetrahedron 34, 505–508 (1970)
Kim, J.H., Thomas, J.J.: Use of 4-(4-nitrobenzyl)pyridine (4-NBP) to test mutagenic potential of slow-reacting epoxides, their corresponding olefins, and other alkylating agents. Bull. Environ. Contam. Toxicol. 49, 879–885 (1992)
Shephard, S.E., Lutz, W.K.: Nitrosation of dietary precursors. Cancer Surv. 8, 401–421 (1989)
García Santos, M.P., Calle, E., Casado, J.: Amino acid nitrosation products as alkylating agents. J. Am. Chem. Soc. 123, 7506–7510 (2001)
García Santos, M.P., González Mancebo, S., Hernández Benito, J., Calle, E., Casado, J.: Reactivity of amino acids in nitrosation reactions and its relation to the alkylating potential of their products. J. Am. Chem. Soc. 124, 2177–2182 (2002)
Pérez Prior, M.T., Manso, J.A., García Santos, M.P., Calle, E., Casado, J.: Reactivity of lactones and GHB formation. J. Org. Chem. 70, 420–426 (2005)
Manso, J.A., Pérez Prior, M.T., García Santos, M.P., Calle, E., Casado, J.: A kinetic approach to the alkylating potential of carcinogenic lactones. Chem. Res. Toxicol. 18, 1161–1166 (2005)
Pérez Prior, M.T., Manso, J.A., García Santos, M.P., Calle, E., Casado, J.: Alkylating potential of potassium sorbate. J. Agric. Food Chem. 53, 10244–10247 (2005)
Tindall, G.W.: Mobile-phase buffers, part I: the interpretation of pH in partially aqueous mobile phases. LCGC North Am. 20, 1028–1032 (2002)
Eklund, T.: The antimicrobial effect of dissociated and undissociated sorbic acid at different pH levels. J. Appl. Bacteriol. 54, 383–389 (1983)
Food Safety & Hygiene, Bulletin for the Australian Food Industry, p. 2 (June 2003)
Connors, K.A.: Chemical Kinetics The Study of Reaction Rates in Solution. VCH, New York (1990), pp. 208 and 246
Exner, O.: Correlation Analysis of Chemical Data. Plenum, New York (1988), Chap. 3
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pérez-Prior, M.T., Manso, J.A., del Pilar García-Santos, M. et al. Sorbic Acid as an Alkylating Agent. J Solution Chem 37, 459–466 (2008). https://doi.org/10.1007/s10953-008-9251-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-008-9251-9