Abstract
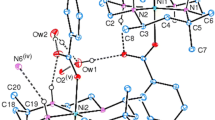
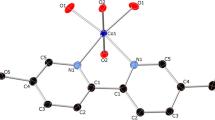
The coordination features of a polyaza macrocycle, containing the diverse bipyridine unit 4,4′-(2,5,6,11,14-pentaaza[15]-[15](2,2′)-bipyridylophane (L3), with Co(II) and Cd(II) have been studied in aqueous solution and in the aprotic solvent dimethylsulfoxide (DMSO). The study was carried out at 298 K by means of potentiometric, spectrophotometric and calorimetric techniques. The formation of the dinuclear species M2 L3 is observed for Co(II) both in water and in DMSO, whereas Cd(II) is able to form this type of dinuclear complex only in DMSO. The FT-IR spectra of the mononuclear species ML3, formed in both solvents, provide evidence that the rigid structure of the polyaminic chain prevents metal ions from being coordinated by all of the nitrogens of the macrocyclic cavity, in good agreement with the behavior suggested by the thermodynamic parameters. The results are compared with those for the complexation of Co(II) and Cd(II) with similar polyazamacrocycles containing a bipyridine unit directly inside the cavity. Semi-empirical calculations were also performed to obtain structural information.
Similar content being viewed by others
References
Bencini, A., Bianchi, A., Giorgi, C., Fusi, V., Masotti, A., Paoletti, P.: Synthesis of polyamine macrocycles and cryptands incorporating bipirydine and phenanthroline moieties. J. Org. Chem. 65, 7686–7689 (2000)
Lehn, J.-M.: Supramolecular Chemistry. VCH, New York (1995)
Lindoy, L.F.: The Chemistry of Macrocyclic Ligand Complexes. Cambridge University Press, Cambridge (1989)
Bradshaw, J.S.: Aza-crown Macrocycles. Wiley, New York (1993)
Gokel, G.W.: Crown Ethers and Cryptands. The Royal Society of Chemistry, Cambridge (1991)
Dobler, M.: Ionophores and Their Structure. Wiley-Interscience, New York (1981)
Izatt, R.M., Bradshaw, J.S., Nielsen, S.A., Lamb, J.D., Christensen, J.J., Sen, D.: Thermodynamic and kinetic data for cation-macrocycle interaction. Chem. Rev. 85, 271–339 (1985)
Izatt, R.M., Pawlak, K., Bradshaw, J.S.: Thermodynamic and kinetic data for macrocycle interactions with cations and anions. Chem. Rev. 91, 1721–2085 (1991)
Bencini, A., Bernardo, M.A., Bianchi, A., Garcia-España, E., Giorgi, C., Luis, S., Pina, F., Valtancoli, B.: Sensing cations and anions by luminescent polyamine receptors in solution. In: Gokel, G.W. (ed.) Advances in Supramolecular Chemistry, vol. 8, pp. 79–130. Cerberus Press, Miami (2002)
Bazzicalupi, C., Bencini, A., Bianchi, A., Danesi, A., Giorgi, C., Lodeiro, C., Pina, F., Santarelli, S., Valtancoli, B.: A Zinc(II)-based receptor for ATP binding and hydrolysis. Chem. Commun. 20, 2630–2632 (2005)
Bianchi, A., Garcia-España, E., Bowman-James, K. (eds.): Supramolecular Chemistry of Anions. Wiley/VCH, New York (1997)
Schneider, H.J.: Mechanisms of molecular recognition—investigations of organic host guest complexes. Angew. Chem. 30, 1417–1436 (1991)
Eliseev, A.V., Schneider, H.J.: Supramolecular chemistry. 46. Molecular recognition of nucleotides, nucleosides, and sugars by aminocyclodextrins. J. Am. Chem. Soc. 116, 6081–6088 (1994) and references cited therein
Beer, P.D., Gale, P.A.: Anion recognition and sensing the state of the art and future perspectives. Angew. Chem. Int. Ed. 40, 486–516 (2001)
Ilioudis, C.A., Steed, J.W.: Polyaza metacyclophanes as ditopic anion receptors. Org. Biomol. Chem. 3, 2935–2945 (2005)
Izatt, R.M., Bradshaw, J.S., Pawlak, K., Bruening, R.L., Tarbet, B.J.: Thermodynamic and kinetic data for macrocycle interaction with neutral molecules. Chem. Rev. 92, 1261–1358 (1992)
Biver, T., Lombardi, D., Secco, F., Tinè, M.R., Venturini, M., Bencini, A., Bianchi, A., Valtancoli, B.: Kinetic and equilibrium studies on the polyazamacrocycle neotetren: metal-complex formation and DNA interaction. Dalton Trans. 8, 1524–1533 (2006)
Anda, C., Bazzicalupi, C., Bencini, A., Bianchi, A., Fornasari, P., Giorgi, C., Valtancoli, B., Lodeiro, C., Parola, A.J., Pina, F.: Cu(II) and Ni(II) complexes with dipyridine-containing macrocyclic polyamines with different binding units. Dalton Trans. 7, 1299–1307 (2003)
Lodeiro, C., Parola, A.J., Pina, F., Bazzicalupi, C., Bencini, A., Bianchi, A., Giorgi, C., Masotti, A., Valtancoli, B.: Protonation and Zn(II) coordination by dipyridine-containing macrocycles with different molecular architecture. A case of pH-controlled metal jumping outside-inside the macrocyclic cavity. Inorg. Chem. 40, 2968–2975 (2001)
Arranz, P., Bazzicalupi, C., Bencini, A., Bianchi, A., Ciattini, S., Fornasari, P., Giorni, C., Valtancoli, B.: Cd(II) and Pb(II) complexation by dipyridine-containing macrocycles with different molecular architecture. Effect of complex protonation on metal coordination environment. Inorg. Chem. 40, 6383–6389 (2001)
Bazzicalupi, C., Bencini, A., Bianchi, A., Del Piero, S., Fornasari, P., Giorgi, C., Melchior, A., Portanova, R., Tolazzi, M., Valtancoli, B.: Co(II) and Cd(II) complexation with two dipyridine-containing macrocyclic polyamines in water and dimethyl sulfoxide. New J. Chem. 29, 805–811 (2005)
Comuzzi, C., Grespan, M., Polese, P., Portanova, R., Tolazzi, M.: Thermodynamic studies on the complexation of cobalt(II) with nitrogen donor ligands in dimethyl sulfoxide. Inorg. Chim. Acta 321, 49–55 (2001)
Cassol, A., Di Bernardo, P., Zanonato, P.L., Portanova, R., Tolazzi, M.: Thermodynamics of complex formation of silver with amines in dimethyl sulfoxide. J. Chem. Soc. Dalton Trans. 3, 657–659 (1987)
Vogel, A.I.: Textbook of Quantitative Chemical Analysis, 5th edn. Longman Group, London (1989)
Gran, G.: Determination of the equivalence point in potentiometric titrations. Analyst 77, 661–671 (1952)
Gans, P., Sabatini, A., Vacca, A.: Investigation of equilibria in solution. Determination of equilibrium constants with the HYPERQUAD suite of programs. Talanta 43, 1739–1753 (1996)
Smith, R.M., Martell, A.E.: NIST Stability Constants Database, Version 4.0. National Institute of Standards and Technology, Washington (1997)
Arnek, R.: High-speed computers as a supplement to graphical methods. II. Application of LETAGROP to calorimetric titrations. Ark. Kemi 32, 81–88 (1970)
Rossotti, F.J.C., Rossotti, H.: The Determination of Stability Constants. McGraw-Hill, New York (1968)
Del Piero, S., Melchior, A., Polese, P., Portanova, R., Tolazzi, M.: A novel multipurpose excel tool for equilibrium speciation based on Newton-Raphson method and on a hybrid genetic algorithm. Ann. Chimica 96(1–2), 29–49 (2006); also on the web, site http://www.freewebs.com/solverstat/est/est.htm
Granovsky, A.: PC-GAMESS version 7.0. http://classic.chem.msu.su/gran/gamess/index.html
Becke, A.D.: A new mixing of Hartree-Fock and local density-functional theories. J. Chem. Phys. 98, 1372–1377 (1993)
Lee, C., Yang, W., Parr, R.G.: Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37, 785–789 (1988)
Hay, P.J., Wadt, W.R.: Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms scandium to mercury. J. Chem. Phys. 82, 270–283 (1985)
Meyerstein, D.: Are M-N bonds indeed inherently weaker when N is a tertiary rather than a primary or secondary nitrogen atom? Coord. Chem. Rev. 185–186, 141–147 (1999)
Odani, A., Masuda, M., Inukai, K., Yamauchi, O.: Pteridine-containing ternary and quaternary complexes as models for metalloenzyme-pterin cofactor-substrate association—structure of ternary copper(II)-2,2′-bipyridine lumazine complex and successful equilibrium study of a quaternary copper(II) system. J. Am. Chem. Soc. 114, 6294–6300 (1992)
Comuzzi, C., Grespan, M., Melchior, A., Portanova, R., Tolazzi, M.: Thermodynamics of complexation of cadmium(II) by open-chain N-donor ligands in dimethyl sulfoxide solution. Eur. J. Inorg. Chem. 12, 3087–3094 (2001)
Comuzzi, C., Melchior, A., Polese, P., Portanova, R., Tolazzi, M.: Cobalt(II) complexes with nitrogen donors and their dioxygen affinity in dimethyl sulfoxide. Eur. J. Inorg. Chem. 8, 2194–2201 (2002)
Mucci, A., Domain, R., Benoit, R.L.: Solvent effect on the protonation of some alkylamines. Can. J. Chem. 58, 953–958 (1980)
Crampton, M.R., Robotham, I.A.: Acidities of some substituted ammonium ions in dimethyl sulfoxide. J. Chem. Res. (S) 1, 22–23 (1997)
Benoit, R.L., Mackinnon, M.J., Bergeron, L.: Basicity of N-substituted anilines and pyridine in dimethyl sulfoxide. Can. J. Chem. 59, 1501–1504 (1981)
Comuzzi, C., Melchior, A., Polese, P., Portanova, R., Tolazzi, M.: Thermodynamics of complex formation of silver(I), cadmium(II) and cobalt(II) with open-chain polyamines in dimethyl sulfoxide and molecular dioxygen binding to cobalt(II) complexes. Eur. J. Inorg. Chem. 10, 1948–1955 (2003)
Del Piero, S., Di Bernardo, P., Fedele, R., Melchior, A., Polese, P., Tolazzi, M.: Affinity of polypyridines towards Cd(II) and Co(II) ions: a thermodynamic and DFT study. Eur. J. Inorg. Chem. 18, 3738–3745 (2006)
Stradsdeit, H., Duhme, A.K., Weber, M., Pohl, S.: Structures of bis(1,4,7-triazacyclononane)cadmium(II) diperchlorate, [Cd(tacn)2](ClO4)2(1), and bis(1,4,7-triazacyclononane)cadmium(II) bis(tetraphenylborate) bis(dimethyl sulfoxide) solvate, [Cd(tacn)2](bPh4)2⋅2(CH3)2SO (2). Acta Crystallogr. Sect. C.: Cryst. Struct. Commun. 48, 437–440 (1992)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bencini, A., Bianchi, A., Del Piero, S. et al. Coordination Features of a Polyaza-Bipyridine-Macrocyclic Ligand toward Co(II) and Cd(II) in Water and Dimethylsulfoxide. J Solution Chem 37, 503–517 (2008). https://doi.org/10.1007/s10953-008-9249-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-008-9249-3