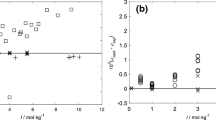
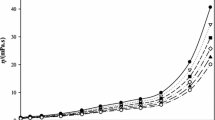
Abstract
Densities of NaOH(aq) solutions with molalities between 0.033 and 6.047 mol⋅kg−1 were measured with a vibrating-tube densitometer at temperatures between 373 K and 623 K and pressures from near the saturation vapor pressure of water to 30 MPa. Apparent molar volumes, V φ, calculated from the measured densities in this work were well represented with the Pitzer ion-interaction treatment up to T = 523 K. Above that temperature the formation of ion pairs must be taken into account to describe correctly the molality dependence of V φ and for the reliable extrapolation of V φ to infinite dilution. Standard-state thermodynamic properties for the ion formation reaction were taken from recently published electrical conductivity measurements. A comparison with previous correlations of volumetric properties of NaOH(aq) is presented covering the full range of pressure and temperature.
Similar content being viewed by others
References
Hayward, A.M., Perman, E.P.: Vapour pressure and heat of dilution. Part VII. Vapour pressures of aqueous solutions of sodium hydroxide and of alcoholic solutions of calcium chloride. Trans. Faraday Soc. 27, 59–69 (1931)
Akerlof, G., Kegeles, G.: The density of aqueous solutions of sodium hydroxide. J. Am. Chem. Soc. 61, 1027–1032 (1939)
Krings, W.: Die Viscosität und die Dichte von Natronlauge bis zu hohen Kozentrationen und bei höheren Temperaturen. Zeit. Anorg. Chem. 255, 294–298 (1948)
Marsh, R.N., Stokes, R.H.: The conductance of dilute sodium hydroxide solutions from 15 °C to 75 °C. Aust. J. Chem. 17, 740–749 (1964)
Roux, A.H., Perron, G., Desnoyers, J.E.: Capacités Calorifiques, Volumes et compressibilités des solutions aqueuses concentrées de LiOH, NaOH et KOH. Can. J. Chem. 62, 878–885 (1984)
Herrington, T.M., Pethybridge, A.D., Roffey, M.G.: Densities of aqueous lithium, sodium, and potassium hydroxides from 25 to 75 °C at 1 atm. J. Chem. Eng. Data 31, 31–34 (1986)
Simonson, J.M., Ryther, R.J.: Volumetric properties of aqueous sodium hydroxide from 273.15 to 348.15 K. J. Chem. Eng. Data 34, 57–63 (1989)
Dibrov, I.A., Mashovets, V.P., Matveeva, R.P.: The density and compressibility of aqueous sodium hydroxide solutions at high temperatures. Zh. Prikl. Khim. 37, 29–36 (1964)
Krumgalz, B.S., Mashovets, V.P.: Density of concentrated NaOH solutions (over 45% by weight) at temperatures up to 400°. Zh. Prikl. Khim. 37, 2596–2600 (1964)
Krey, J.: Dampfdruck und Dichte des Systems H2O-NaOH. Z. Physik. Chem. Neue Folge 81, 252–273 (1972)
Pabalan, R.T., Pitzer, K.S.: Thermodynamics of NaOH(aq) in hydrothermal solutions. Geochim. Cosmochim. Acta 51, 829–837 (1987)
Simonson, J.M., Mesmer, R.E., Rogers, P.S.Z.: The enthalpy of dilution and apparent molar heat capacity of NaOH(aq) to 523K and 40MPa. J. Chem. Thermodyn. 21, 561–584 (1989)
Pitzer, K.S.: Thermodynamics of electrolytes I. Theoretical basis and general equations. J. Phys. Chem. 77, 268–277 (1973)
Pitzer, K.S.: Ion Interaction Approach: Theory and Data Correlation, In Activity Coefficients in Electrolyte Solutions, Chap. 3, R.Pytkowicz, Ed. pp. 157–208, CRC Press, Boca Raton (1979).
Corti, H.R., Fernandez-Prini, R., Svarc, F.E.: Densities and partial molar volumes of aqueous solutions of lithium, sodium, and potassium hydroxides up to 250 °C. J. Solution Chem 19, 793–809 (1990)
Kerschbaum, S., Franck, E.U.: High pressure supercritical PVT-data of water-sodium hydroxide mixtures and of liquid sodium hydroxide to 673K and 400MPa. Ber. Bunsenges. Phys. Chem. 99, 624–632 (1995)
Eberz, A., Franck, E.U.: High pressure electrolyte conductivity of the homogeneous, fluid water-sodium hydroxide system to 400 °C and 3000 bar. Ber. Bunsenges. Phys. Chem. 99, 1091–1103 (1995)
Abdulagatov, I.M., Dvoryanchikov, V.I., Mursalov, B.A., Kamalov, A.N.: Measurements of heat capacities at constant volume for aqueous salt solutions (H2O + NaCl, H2O + KCl and H2O + NaOH) near the critical point of pure water. Fluid Phase Equil. 143, 213–239 (1998)
Ho, P.C., Palmer, D.A.: Ion association of dilute aqueous sodium hydroxide solutions to 600 °C and 300MPa by conductance measurements. J. Solution Chem. 25, 711–729 (1996)
Ho, P.C., Palmer, D.A., Wood, R.H.: Conductivity measurement of dilute aqueous LiOH, NaOH, and KOH solutions at high temperatures and pressures using a flow-through cell. J. Phys. Chem. B 104, 12084–12089 (2000)
Simonson, J.M., Oakes, C.S., Bodnar, R.J.: Densities of NaCl(aq) to the temperature 523K at pressures to 40MPa measured with a new vibrating-tube densitometer. J. Chem. Thermodyn. 26, 345–359 (1994)
Hill, P.G.: A unified fundamental equation for the thermodynamic properties of H2O. J. Phys. Chem. Ref. Data 19 1233–1274 (1990)
Majer, V., Gates, J.A., Inglese, A., Wood, R.H.: Volumetric properties of aqueous NaCl solutions from 0.0025 to 5.0molċkg-1, 323 to 600K, and 0.1 to 40MPa. J. Chem. Thermodyn. 20, 949–968 (1988)
Simonson, J.M., Oakes, C.S.: unpublished results.
Archer, D.G., Wang, P.: The dielectric constant of water and Debye–Hückel limiting law slopes. J. Phys. Chem. Ref. Data 19, 371–410 (1990)
Majer, V., Wood, R.H.: Volumetric properties of aqueous 1–1 electrolytes near and above the critical temperature of water. III. Experimental densities and apparent molar volumes of CsBr(aq) to the temperature 725.5K and the pressure 38.0MPa, comparison with other 1–1 electrolytes, and extrapolations to infinite dilution for NaCl(aq). J. Chem. Thermodyn. 26, 1143–1166 (1994)
Sharygin, A.V., Wood, R.H.: Volumes and heat capacities of aqueous solutions of hydrochloric acid at temperatures from 298.15K to 623K and pressures to 28MPa. J. Chem. Thermodyn. 29, 125–148 (1997)
Obšil, M., Majer, V., Hefter, G.T., Hynek, V.: Volumes of MgCl2(aq) at temperatures from 298K to 623K and pressures up to 30MPa. J. Chem. Thermodyn. 29, 575–593 (1997)
Bianchi, H., Corti, H.R., Fernandez-Prini, R.: Electrical conductivity of aqueous sodium hydroxide at high temperatures. J. Solution Chem. 23, 1203–1212 (1994)
Shock, E.L., Helgeson, H.C.: Calculation of the thermodynamic and transport properties of aqueous species at high pressures and temperatures: Correlation algorithms for ionic species and equation of state predictions to 5kb and 1000 °C. Geochim. Cosmochim. Acta 52, 2009–2036 (1988)
Mesmer, R.E., Marshall, W.L., Palmer, D.A., Simonson, J.M., Holmes, H.F.: Thermodynamics of aqueous association and ionization reactions at high temperatures and pressures. J. Solution Chem. 17, 699–718 (1988)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Corti, H.R., Simonson, J.M. Densities and Apparent Molar Volumes of NaOH(aq) to the Temperature 623 K and Pressure to 30 MPa. J Solution Chem 35, 1057–1074 (2006). https://doi.org/10.1007/s10953-006-9054-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-006-9054-9