Abstract
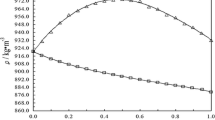
The densities of binary systems of difurylmethane (DFM) in methanol have been measured with an Anton Parr DMA 4500 vibrating-tube densimeter over the entire composition range at intervals of 5 K in the temperature range between 288.15 and 308.15 K. Excess molar volumes of the mixture, apparent molar volumes of DFM, and excess partial molar volumes of both components have been calculated to provide insight into the intermolecular interaction present in the mixtures investigated. Excess molar volumes have been fitted to a Redlich–Kister equation and they exhibited negative deviations from ideal behavior. Both the apparent molar volume of DFM and excess partial molar volumes of DFM and methanol exhibit a dependence on composition but are less sensitive to temperature.
Similar content being viewed by others
References
G. C. Benson and O. Kiyohara, J. Solution Chem. 9, 791 (1980).
M. I. Davis, Chem. Soc. Rev. 22, 127 (1993).
G. Douhéret and M. I. Davis, Chem. Soc. Rev. 22, 43 (1993).
M. J. Blandamer, Chem. Soc. Rev. 27, 73 (1998).
J. R. Dixon, W. O. George, Md. F. Hossain, R. Lewis, and M. Price, J. Chem. Soc., Faraday Trans. 20, 3611 (1997).
S. Scheiner, Hydrogen Bonding (Oxford University Press, New York, 1997).
O. Redlich and T. A. Kister, Ind. Eng. Chem. 40, 345 (1948).
R. N. French and C. M. Criss, J. Solution Chem. 10, 231 (1981).
H. Ogawa and S. Murakami, J. Solution Chem. 16, 315 (1987).
D. Hamiliton and R. H. Stokes, J. Solution Chem. 1, 213 (1972).
I. M. S. Lampreia and E. F. G. Barbosa, Fluid Phase Equilib. 71, 125 (1992).
E. F. G. Barbosa and I. M. S. Lampreia, Can. J. Chem. 64, 387 (1986).
A. H. Fawcett and W. Ddamba, Makromol. Chem. 183, 2799 (1982).
A. H. Fawcett, T. F. Yau, J. N. Mulemwa, and C. E. Tan, Br. Polym. J. 19, 211 (1987).
S. L. Buchwalter, J. Polym. Sci., Polym. Chem. Ed. 23, 287 (1985).
Handbook of Chemistry and Physics (CRC Press, Boca Raton, FL, 1982–1983), p. F5; Anton Parr DMA 4500/5000 Instruction Manual, (Graz, Austria, 2001), p. 97; A. Petek and V. Doleek, Acta Chim. Slov. 45, 153 (1998).
P. Bevington, Data Reduction and Error Analysis for Physical Sciences (McGraw Hill, New York, 1969), p. 200.
W. E. Acree Jr., Thermodynamic Properties of Nonelectrolytes (Academic Press, Orlando, FL, 1984); Y. Mahan, T. T. Teng, L. G. Hepler, and A. E. Mather, J. Solution Chem. 23, 195 (1994).
B. Orge, M. Iglesias, G. Marino, and J. Tojo, J. Chem. Thermodyn. 31, 497 (1999).
B. Lee, J. Phys. Chem. 87, 112 (1983).
I. M. S. Lampreia and L. A. V. Ferreira, J. Chem. Soc., Faraday Trans. 92, 47 (1993).
P. A. Leduc, J. C. Fortier, and J. E. Desnoyers, J. Phys. Chem. 78, 1217 (1974).
J. F. Reading, I. D. Watson, and G. R. Hedwig, J. Chem. Thermodyn. 22, 159 (1990).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mokate, O., Ddamba, W.A.A. Volumetric Properties of Difurylmethane in Methanol from 288.15 to 308.15 K. J Solution Chem 34, 1327–1339 (2005). https://doi.org/10.1007/s10953-005-8023-z
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10953-005-8023-z