Abstract
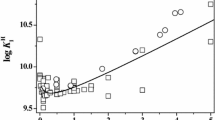
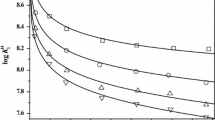
The constants for the dissociation of citric acid (H3C) have been determined from potentiometric titrations in aqueous NaCl and KCl solutions and their mixtures as a function of ionic strength (0.05–4.5 mol-dm−3) at 25 °C. The stoichiometric dissociation constants (K i *)
were used to determine Pitzer parameters for citric acid (H3C), and the anions, H2C−, HC2−, and C3−. The thermodynamic constants (K i ) needed for these calculations were taken from the work of R. G. Bates and G. D. Pinching (J. Amer. Chem. Soc. 71, 1274; 1949) to fit to the equations (T/K):
The values of Pitzer interaction parameters for Na+ and K+ with H3C, H2C−, HC2−, and C3− have been determined from the measured pK values. These parameters represent the values of pK1*, pK2*, and pK3*, respectively, with standard errors of σ = 0.003–0.006, 0.015–0.016, and 0.019–0.023 for the first, second, and third dissociation constants. A simple mixing of the pK* values for the pure salts in dilute solutions yield values for the mixtures that are in good agreement with the measured values. The full Pitzer equations are necessary to estimate the values of pK i * in the mixtures at high ionic strengths. The interaction parameters found for the mixtures are ΨNa-K − H2C = − 0.00823 ± 0.0009; ΨNa-K − HC = − 0.0233 ± 0.0009, and ΨNa-K − C = 0.0299 ± 0.0055 with standard errors of σ(pK1) = 0.011, σ(pK2) = 0.011, and σ(pK3) = 0.055.
Similar content being viewed by others
References
W. R. Harris, Z. Wang, C. Brook, and A. Islam, (2003) Inorg. Chem. 42, 5880.
J. P. Chen, S. Wu, and K.-H. Chong, (2003) Carbon 41, 1979.
R.-S. Juang, S.-H. Lin, and T.-Y. Wang, (2003) Chemosphere 53, 1221.
X.-Q. Shan, J. Lian, and B. Wen, (2002) Chemosphere 47, 701.
J. Mizera, A. H. Bond, G. R. Choppin, and R. C. Moore, Actnide Speciation in High Ionic Media, D. T. Reed, S. Clark, and L. Rao, eds. (Plenum Publishing, New York, 1999), 113 pp.
P. G. Daniele, A. De Robertis, C. De Stefano, A. Gianguzza, and S. Sammartano, (1990) J. Chem. Res. (S) 300(M), 2316.
A. De Robertis, C. De Stefano, C. Rigano, and S. Sammartano, (1990) J. Solution Chem. 19, 569.
C. Foti, A. Gianguzza, and S. Sammartano, (1997) J. Solution Chem. 26, 631.
A. De Robertis, C. DeStefano, and C. Foti, (1999) J. Chem. Eng. Data 44, 262.
R. G. Bates and G. D. Pinching, (1949) J. Am. Chem. Soc. 71, 1274.
P. Bénézeth, D. A. Palmer, and D. J. Wesolowski, (1997) J. Solution Chem. 26, 63.
J. A. Schwarz, C. Contescu, V. T. Popa, A. Contescu, and Y. Lin, (1996) J. Solution Chem. 25, 877.
C. De Stefano, C. Foti, A. Gianguzza, and S. Sammartano, (2000) Mar. Chem. 72, 61.
C. De Stefano, P. Princi, C. Rigano, and S. Sammartano, (1987) Ann. Chim. (Rome) 77, 643.
A. LoSurdo, E. M. Alzola, and F. J. Millero, (1982) J. Chem. Thermodyn. 14, 649.
E. Dedick, D. J. Stade, S. Sotolongo, J. P. Hershey, and F. J. Millero, J. Solution Chem. 19, 353 (1989).
K. S. Pitzer, Activity Coefficients in Electrolyte Solutions, Vol. I R. M. Pytkowicz, ed. (CRC Press. Boca Raton, FL, 1979), pp. 157–208.
F. J. Millero and D. Pierrot, Aquatic Geochem. 4, 153 (1998).
N. Mø ller, Geochim. Cosmochim. Acta 52, 821–837 (1988).
H. S. Harned and B. B. Owen, The Physical Chemistry of Electrolytic Solutions, 3rd edn. ACS Monograph No. 137 (Reinhold, New York, 1958), 803 pp.
T. F. Young, Rec. Chem. Progr. 12, 81 (1951).
F. J. Millero, Physical Chemistry of Natural Waters (Wiley-Interscience, New York, 2001), 654 pp.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Crea, F., De Stefano, C., Millero, F.J. et al. Dissociation Constants for Citric Acid in NaCl and KCl Solutions and their Mixtures at 25 °C. J Solution Chem 33, 1349–1366 (2004). https://doi.org/10.1007/s10953-004-1046-z
Issue Date:
DOI: https://doi.org/10.1007/s10953-004-1046-z