Abstract
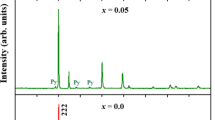
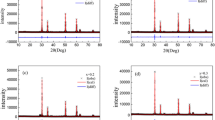
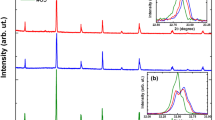
The present article reviews the structural and magnetic properties of Y2Zr2−xMnxO7 compounds with respect to the extent of Mn doping. All the samples are single phase and crystallize in the cubic system with Fd-3m space group. The stability of the pyrochlore structure was established by Rietveld structural refinements. The lattice parameter and cell volume decrease with Mn substitution because of the smaller ionic radius of Mn4+ than Zr4+. The zero field-cooled (ZFC) and field-cooled (FC) curves for Mn-doped samples diverge at low temperature which is possibly due to spin-glass transition. Both the Mn-doped phases exhibit anti-ferromagnetic behavior, which could be due to the presence of super-exchange (SE) Mn4+–O2−–Mn4+ interactions. Moreover, small magnetic hysteresis loops are observed for the Mn-doped phases suggesting the presence of weak ferromagnetic interactions.







Similar content being viewed by others
References
Chamberlain, S.L., Hess, S.T., Corruccini, L.R.: Dipolar magnetic order in the pyrochlore structure. Phys. Lett. A. 323, 310–314 (2004)
Raju, N.P., Dion, M., Gingras, M.J.P., Mason, T.E., Greedan, J.E.: Transition to long-range magnetic order in the highly frustrated insulating pyrochlore antiferromagnet Gd2Ti2O7. Phys. Rev. B. 59, 14489–14498 (1999)
Lian, J., Wang, L.M., Wang, S.X., Chen, J., Boatner, L.A., Ewing, R.C.: Nanoscale manipulation of pyrochlore: new nanocomposite ionic conductors. Phys. Rev. Lett. 87(1–4), 145901 (2001)
Poulsen, F.W., Glerup, M., Holtappels, P.: Structure, Raman spectra and defect chemistry modelling of conductive pyrochlore oxides. Solid State Ionics. 135, 595–602 (2000)
Wilde, P.J., Catlow, C.R.: Molecular dynamics study of the effect of doping and disorder on diffusion in gadolinium zirconate. Solid State Ionics. 112, 185–195 (1998)
Dong, X.W., Wang, K.F., Luo, S.J., Wan, J.G., Liu, J.M.: Coexistence of magnetic and ferroelectric behaviors of pyrochlore Ho2Ti2O7. J. Appl. Phys. 106(1–4), 104101 (2009)
Mirsaneh, M., Hayden, B.E., Furman, E., Perini, S., Lanagan, M.T., Reaney, I.M.: High dielectric tunability in lead niobate pyrochlore films. Appl. Phys. Lett. 100(1–3), 082901 (2012)
Wang, S.X., Wang, L.M., Ewing, R.C., Kutty, K.G.: Ion irradiation of rare-earth and yttrium-titanate-pyrochlores. Nucl. Inst. Methods Phys. Res. B. 169, 135–140 (2000)
Reid, D.P., Stennett, M.C., Hyatt, N.C.: The fluorite related modulated structures of the Gd2(Zr2−xCex)O7 solid solution: an analogue for Pu disposition. J. Solid State Chem. 191, 2–9 (2012)
Chen, Z.S., Gong, W.P., Chen, T.F., Li, S.L.: Synthesis and characterization of pyrochlore-type yttrium titanate nanoparticles by modified sol–gel method. Bull. Mater. Sci. 34, 429–434 (2011)
Moon, P.K., Tuller, H.L.: Ionic conduction in the Gd2Ti2O7−Gd2Zr2O7 system. Solid State Ionics. 28, 470–474 (1988)
Subramanian, M.A., Aravamudan, G., Rao, G.S.: Oxide pyrochlores—a review. Prog. Solid State Chem. 15, 55–143 (1983)
Wang, J., Nakamura, A., Takeda, M.: Structural properties of the fluorite-and pyrochlore-type compounds in the Gd2O3–ZrO2 system xGdO1.5–(1−x)ZrO2 with 0.18≤ x≤ 0.62. Solid State Ionics. 164, 185–191 (2003)
Mandal, B.P., Garg, N., Sharma, S.M., Tyagi, A.K.: Preparation, XRD and Raman spectroscopic studies on new compounds RE2Hf2O7 (RE= Dy, Ho, Er, Tm, Lu, Y): pyrochlores or defect-fluorite? J. Solid State Chem. 179, 1990–1994 (2006)
Muromura, T., Hinatsu, Y.: Fluorite type phase in nuclear waste ceramics with high zirconia and alumina contents. J. Nucl. Mater. 151, 55–62 (1987)
Hayakawa, I., Kamizono, H.: Investigation of waste form materials suitable for immobilizing actinide elements in high-level waste. Mater. Res. Soc. Symp. Proc. 257, 257 (1992)
Mandal, B.P., Tyagi, A.K.: Preparation and high temperature-XRD studies on a pyrochlore series with the general composition Gd2−xNdxZr2O7. J. Alloys Compd. 437, 260–263 (2007)
Shinozaki, K., Miyauchi, M., Kuroda, K., Sakurai, O., Mizutani, N., Kato, M.: Oxygen-ion conduction in the Sm2Zr2O7 pyrochlore phase. J. Am. Ceram. Soc. 62, 538–539 (1979)
Sayed, F.N., Jain, D., Mandal, B.P., Pillai, C.G., Tyagi, A.K.: Tunability of structure from ordered to disordered and its impact on ionic conductivity behavior in the Nd2−yHoyZr2O7 (0.0≤ y ≤2.0) system. RSC Adv. 2, 8341–8351 (2012)
Lehmann, H., Pietzer, D., Pracht, G., Vassen, R., Stover, D.: Thermal conductivity and thermal expansion coefficients of the lanthanum rare-earth-element zirconate system. J. Am. Ceram. Soc. 86, 1338–1344 (2003)
Thampi, D.V., Rao, P.P., Radhakrishnan, A.N.: Influence of Ce substitution on the order-to-disorder structural transition, thermal expansion and electrical properties in Sm2Zr2−xCexO7 system. RSC Adv. 4, 12321–12329 (2014)
Gao, B., Chen, T., Tam, D.W., Huang, C.L., Sasmal, K., Adroja, D.T., Ye, F., Cao, H., Sala, G., Stone, M.B., Baines, C.: Experimental signatures of a three-dimensional quantum spin liquid in effective spin-1/2Ce2Zr2O7 pyrochlore. Nat. Phys. 15, 1052–1057 (2019)
Reimers, J.N., Greedan, J.E., Kremer, R.K., Gmelin, E., Subramanian, M.A.: Short-range magnetic ordering in the highly frustrated pyrochlore Y2Mn2O7. Phys. Rev. B. 43, 3387–3394 (1991)
Kennedy, B.J., Hunter, B.A.: Structural and magnetic studies of manganese-containing pyrochlore oxides. J. Alloys Compd. 302, 94–100 (2000)
Raju, N.P., Greedan, J.E., Subramanian, M.A.: Magnetic, electrical, and small-angle neutron-scattering studies of possible long-range order in the pyrochlores Tl2Mn2O7 and In2Mn2O7. Phys. Rev. B. 49, 1086–1091 (1994)
Liang, D., Liu, H., Ling, L., Zhang, L., Zhang, C., Zhang, Y.: Magnetic and magnetoelectric properties of hybrid-frustrated Bi2Ir2−xMnxO7 pyrochlores. Solid State Commun. 278, 36–41 (2018)
Shimakawa, Y., Kubo, Y., Manako, T.: Giant magnetoresistance in Ti2Mn2O7 with the pyrochlore structure. Nature. 379, 53–55 (1996)
Kumar, M., Raj, I.A., Pattabiraman, R.: Y2Zr2O7 (YZ)-pyrochlore based oxide as an electrolyte material for intermediate temperature solid oxide fuel cells (ITSOFCs)—influence of Mn addition on YZ. Mater. Chem. Phys. 108, 102–108 (2008)
Jain, S.R., Adiga, K.C., Verneker, V.P.: A new approach to thermochemical calculations of condensed fuel-oxidizer mixtures. Combust. Flame. 40, 71–79 (1981)
Bhattachaya, A.K., Hartridge, A., Mallick, K.K., Woodhead, J.L.: Preparation and characterization of Ln2Zr2O7 microspheres by an inorganic sol-gel route. J. Mater. Sci. 29, 6076–6078 (1994)
Larson, A.C., Von Dreele, R.B.: General Structure Analysis System (GSAS), Los Alamos National Laboratory Report LAUR. 86–748 (2004)
Ewing, R.C., Chakoumakos, B.C., Cerny, P.: Granitic Pegmatites in Science and Industry: a Short Course, Mineral Association of Canada 239–265 (1982)
Chakoumakos, B.C.: Systematics of the pyrochlore structure type, ideal A2B2X6Y. J. Solid State Chem. 53, 120–129 (1984)
Chakoumakos, B.C., Ewing, R.C.: Crystal chemical constraints on the formation of actinide pyrochlores. Mater. Res. Soc. Proc. 44, 641–646 (1984)
Subramanian, M.A., Sleight, A.W.: Rare earth pyrochlores. Handbook on the Physics and Chemistry of Rare Earths 16, 225–248 (1993)
Klug, H.P., Alexander, L.E.: X-ray Diffraction Procedures; for Polycrystalline and Amorphous Materials. Wiley, Toronto (1954)
Feng, Y., Zhu, S., Bian, J., Chen, F., Chen, S., Ma, C., Liu, H., Fang, B.: Magnetic and electrical transport properties of the pyrochlore iridate Bi2-xCoxIr2O7. J. Magn. Magn. Mater. 451, 283–287 (2018)
Haas, M.K., Cava, R.J., Avdeev, M., Jorgensen, J.D.: Robust paramagnetism in Bi2−xMxRu2O7 (M = Mn, Fe, Co, Ni, Cu) pyrochlore. Phys. Rev. B. 66(1–7), 094429 (2002)
Kittel, C., McEuen, P.: Introduction to Solid State Physics, pp. 404–406. Wiley, New York (1986)
Singh, D., Mahajan, A.: Effect of A-site cation size on the structural, magnetic, and electrical properties of La1−xNdxMn0.5Cr0.5O3 perovskites. J. Alloys Compd. 644, 172–179 (2015)
Sharma, N.D., Sharma, S., Choudhary, N., Verma, M.K., Singh, D.: Comparative study of La0.5Nd0.2Ca0.3−xKxMnO3 (x= 0.0 and 0.05) nanoparticles: effect of A-cation size and calcination temperature. Ceram. Int. 45, 13637–13646 (2019)
Zhu, X., Edström, A., Ederer, C.: Magnetic exchange interactions in SrMnO3. Phys. Rev. B. 101(1–10), 064401 (2020)
Acknowledgments
Authors are obliged to Director, AMRC, IIT Mandi, for XRD analysis. Special thanks to Prof. R. Chandra, IIC, IIT Roorkee, for SEM and EDX analyses and Director, IISER, Bhopal, for carrying out magnetic measurements.
Funding
CSIR, New Delhi, provided financial assistance, vide Ref. No. 09/100(0189)/2015-EMR-I.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Verma, M.K., Sharma, S., Choudhary, N. et al. Evolution of Crystal Structure and Magnetic Properties of Y2Zr2−xMnxO7 (x = 0.0, 0.1, 0.2) Family of Pyrochlore Oxides. J Supercond Nov Magn 34, 435–441 (2021). https://doi.org/10.1007/s10948-020-05678-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-020-05678-w