Abstract
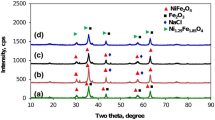
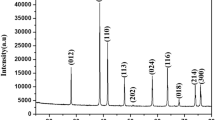
In the present work, nickel-doped iron oxide (NixFe3−x O 4) nanoparticles with different concentration of nickel (x = 0, 0.05, 0.1, and 0.15) have been prepared by co-precipitation method. These prepared nanoparticles have been characterized by using x-ray diffractometer, thermo gravimetric analysis and differential scanning calorimetry, Fourier transform infrared spectroscopy, scanning electron microscopy, vibrating sample magnetometer, and UV-Visible spectroscopy to study their structural, thermal, morphological, magnetic, and optical properties, respectively. The x-ray diffraction confirms the formation of single-phase inverse spinel cubic structure of NiFe3 O 4 nanoparticles. Crystallite size has been estimated by the full width at half maximum of the most intense x-ray diffraction peak where vibrational and stretching modes of metal-oxygen bonds in 872 cm are shown in Fourier transform infrared spectra which confirms the formation of nanoparticles. The thermal analysis revealed that the transition temperature and stability increases with increasing Ni concentration. The surface morphology indicated that the particles are spherical in shape with some agglomeration. The magnetic measurement revealed that the coercivity and anisotropy increases with nickel doping in magnetite nanoparticles. The optical analysis revealed that direct and indirect both types of band gap increases when the particle size decreases because the absorption spectra shift toward smaller wavelength. The blue shift confirms the formation of nanoparticles.
















Similar content being viewed by others
References
Kang, Y., Zhou, L., Li, X., Yuan, J.: Cyclodextrin-modified hybrid magnetic nanoparticles for catalysis and adsorption. J. Mater. Chem. 21(11), 3704–10 (2011)
Barreto, C. H., Santiago, V. R., Freire, R. M., Mazzetto, S. E., Denardin, J. C., Mele, G., et al.: Magnetic nanosystem for cancer therapy using oncocalyxone A, an antitomour secondary metabolite isolated from a Brazilian plant. Int. J. Mol. Sci. 14(9), 18269–83 (2013)
Czichos, H., Saito, T., Smith, L. E.: Springer handbook of metrology and testing. Springer, Berlin Heidelberg (2011)
Schodek, D. L., Ferreira, P., Ashby, M. F.: Nanomaterials, nanotechnologies and design: an introduction for engineers and architects. Elsevier Science (2009)
Fulekar, M. H.: Nanotechnology: importance and applications. I.K. International Publishing House (2010)
Sahay, K., Shivendra Pathak K. S.: Basic concepts of electrical engineering. New Age International (P.) Limited. Publishers (2006)
Chen, C. W.: Magnetism and metallurgy of soft magnetic materials. Dover Publications (1977)
Fuller, A. J. B.: Institution of electrical engineers. Ferrites at microwave frequencies. P. Peregrinus (1987)
Pillai, S. O.: Modern physics and solid state physics (problems and solutions). New Age International (P) Limited (2008)
Smallman, R. E., Bishop, R. J.: Modern physical metallurgy and materials engineering: science, process, applications. Butterworth-Heinemann (1999)
Wei, Y., Han, B., Hu, X., Lin, Y., Wang, X., Deng, X.: Synthesis of Fe3 O 4 nanoparticles and their magnetic properties. Prod. Eng. 27, 632–7 (2012)
Mart, I. -M. I., Espinosa, P. M. E., Rez HíR, P., Arenas-Alatorre, J.: Synthesis of magnetite (Fe3O4) nanoparticles without surfactants at room temperature. Mater. Lett. 61(23GÇô24), 4447–51 (2007)
Larumbe, S.: CG-PJIPLAG-PJAMLFGaDC, Ni doped Fe3 O 4 magnetic nanoparticles. Journal of nanoscience and nanotechnology (2012)
Suryanarayana, C.: Mechanical alloying and milling. CRC Press (2004)
Trindade, T., da Silva, A. L. D.: Nanocomposite particles for bio-applications: materials and bio-interfaces. Pan Stanford (2011)
Petcharoen, K., Sirivat, A.: Synthesis and characterization of magnetite nanoparticles via the chemical co-precipitation method. Mater. Sci. Eng. B 177(5), 421–7 (2012)
Nogi, K., Hosokawa, M., Naito, M., Yokoyama, T.: Nanoparticle technology handbook. Elsevier (2012)
Rahman, O. U., Mohapatra, S. C., Ahmad, S.: Fe3 O 4 inverse spinal super paramagnetic nanoparticles. Mater. Chem. Phys. 132(1), 196–202 (2012)
Akademia. S. R.: Chemical Society Russian Journal of Physical Chemistry. Chemical Society (2006)
Patange, S. M., Shirsath, S. E., Jadhav, S. S., Jadhav, K. M.: Cation distribution study of nanocrystalline NiFe2−xCrx O 4 ferrite by XRD, magnetization and Mossbuar spectroscopy. Applications and material science (2011)
Fox, A. M.: Optical properties of solids. Oxford University Press (2001)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Anjum, S., Saleem, H., Rasheed, K. et al. Role of Ni2+ Ions in Magnetite Nano-particles Synthesized by Co-precipitation Method. J Supercond Nov Magn 30, 1177–1186 (2017). https://doi.org/10.1007/s10948-016-3832-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-016-3832-4