Abstract
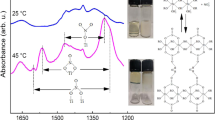
Oxyhydrate gels have a hydrophilic surface, due to which they undergo destruction and secondary polymerization in aqueous media. Prolonged storage in aqueous solution gives rise to regions with selfsimilar helical ordering in gels. Structuring of this kind is also observed when synthesis is conducted under conditions that provide low gelation rates. Electromagnetic UV and visible radiation is another means to change the gel structure; it makes the oligomer species pass into the excited state, due to which one of the directions of structuring becomes dominant. This work summarizes the results of computer simulation of gel agglomerates. For oxyhydrate systems, helical ordering was found to be one of the local energy minima. The units of a macrohelix can lie at various angles relative to one another, and they can change, after absorption of energy, the helix pitch and the order of elements in the helix.
Similar content being viewed by others
References
Yu. V. Egorov, Sorption Statics of Microcomponents with Oxyhydrates [in Russian], Atomizdat, Moscow (1975).
S. I. Pechenyuk, Izv. Ross. Akad. Nauk, No. 2, 229–238 (1999).
S. I. Pechenyuk, L. P. Kuzmich, S. I. Matveenko, and E. V. Kalinkina, Colloid Surf. A: Physicochem. Eng. Aspects, 144, 43–48 (1998).
B. V. Glushkova, Polymorphism of Rare-Earth Oxides [in Russian], Nauka, Leningrad (1967).
V. P. Chalyi, Metal Hydroxides [in Russian], Naukova Dumka, Kiev (1972).
C. Sancher and J. Livage, New J. Chem., 14, Nos. 6/7, 513–521 (1990).
Yu. I. Sukharev, V. V. Avdin, A. A. Lymar, et al., J. Struct. Chem., 47, No. 1, 151–155 (2006).
N. I. Gelperin and G. A. Nosov, Fundamentals of the Fractional Crystallization Technique [in Russian], Khimiya, Moscow (1986).
M. A. Grishina, E. V. Bartashevich, V. A. Potemkin, and A. V. Belik, J. Struct. Chem., 43, No. 6, 1040–1044 (2002).
Yu. I. Sukharev, A. A. Lymar, and V. V. Avdin, Izv. Chelyab. Nauch. Tsentra, Ural Branch, Russian Academy of Sciences, No. 4, 53–57 (2001). (http://csc.ac.ru/news/2001_4/2001_4_10_3. pdf).
Yu. I. Sukharev, V. V. Avdin, T. G. Krupnova, and V. A. Kuznetsova, ibid., No. 2, 67–71 (2000). (http://csc.ac.ru/news/2000_2/2000_2_9_3._pdf).
V. V. Avdin, Yu. I. Sukharev, T. V. Mosunova, and E. A. Nikitin, ibid., No. 2, 68–73 (2003). (http://csc.ac.ru/news/2003_2/2003_2_10_3._zip).
V. V. Avdin, Yu. I. Sukharev, and A. V. Batist, ibid., No. 1, 50–55 (2006). (http://csc.ac.ru/ej/file/1844).
A. A. Lymar, “Quantum-chemical modeling of formation of zirconium oxyhydrates,” Chemical Sciences Candidate’s Dissertation, South Urals State University, Chelyabinsk (2003).
V. V. Avdin, A. V. Batist, and A. A. Lymar, Sorption and Chromatographic Processes, Vol. 6, Pt. 3, pp. 1104–1109 (2006).
V. V. Avdin and A. V. Batist, ibid., Pt. 3, pp. 1157–1162.
Author information
Authors and Affiliations
Corresponding author
Additional information
__________
Translated from Zhurnal Strukturnoi Khimii, Vol. 48, No. 4, pp. 796–801, July–August, 2007.
Original Russian Text Copyright © 2007 by V. V. Avdin, A. A. Lymar, A. V. Batist, E. A. Nikitin, M. Yu. Belkanova, and V. A. Potemkin
Rights and permissions
About this article
Cite this article
Avdin, V.V., Lymar, A.A., Batist, A.V. et al. Structure formation in heavy metal oxyhydrates at low rates of gel formation. J Struct Chem 48, 747–752 (2007). https://doi.org/10.1007/s10947-007-0114-9
Received:
Issue Date:
DOI: https://doi.org/10.1007/s10947-007-0114-9