Abstract
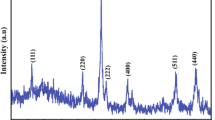
The study is devoted to the size analysis of lead and cadmium nanoparticles prepared by chemical deposition from aqueous medium at 325 K. The coherent scattering region size coincides with the size of particles formed by chemical bath deposition. Therefore, the diffraction technique was applied to determine particle size. The study indicates that lead sulfide particles have B1 structure, and their size increases monotonously from 100 nm to 300 nm with increasing chemical affinity of sulfide formation from 31 kJ/mol to 39 kJ/mol. The size of cadmium sulfide particles varies from 2 nm to 10 nm and does not actually change with variations in chemical affinity over the range of 52–66 kJ/mol. It is established that cadmium sulfide particles smaller than 5 nm have a cubic structure B3, while those larger than 5 nm exhibit an equilibrium hexagonal structure B4.
Similar content being viewed by others
References
A. I. Gusev, and A. A. Rempel, Nanocrystalline materials [in Russian], Nauka, Moscow (2000).
A. P. Alivisatos, Science, 271, No. 5251, 933–937 (1996).
D. L. Klein, R. Roth, A. K. L. Lim, et al., Nature, 389, No. 6652, 699–701 (1997).
V. A. Fonoberov, E. P. Pokatilov, and A. A. Balandin, Phys. Rev. B, 66, 085310-1–085310-13 (2002).
F. W. Wise, Acc. Chem. Res., 33, No. 11, 773–780 (2000).
Z. Hens, D. Vanmaekelbergh, E. J. A. J. Stoffels, and H. van Kempen, Phys. Rev. Lett., 88, No. 23, 236803-1–236803-4 (2002).
A. R. Kortan, R. Hull, R. L. Opila, et al., J. Am. Chem. S., 112, No. 4, 1327–1332 (1990).
P. O’Brien and J. McAleese, J. Mat. Chem., 8, No. 11, 2309–2314 (1998).
N. S. Belova, A. A. Uritzkaya, and G. A. Kitaev, Zh. Prikl. Khim., 75, No. 10, 1598–1601 (2002).
B. E. Warren, X-Ray Diffraction, Dover Publications, New York (1990).
R. W. James, The Optical Principles of the Diffraction of X-rays, Ox Bow Press, Woodbridge (Connecticut) (1982), p. 664. (reprint of 1962 edition).
L. Pasquini, A. A. Rempel, R. Würschum, et al., Phys. Rev. B, 63, No. 13, 134114-1–134114-7 (2001).
Author information
Authors and Affiliations
Additional information
Original Russian Text Copyright © 2004 by N. S. Kozhevnikova, A. S. Kurlov, A. A. Uritzkaya, and A. A. Rempe
__________
Translated from Zhurnal Strukturnoi Khimii, Vol. 45, Supplement, pp. 156–162, 2004.
Rights and permissions
About this article
Cite this article
Kozhevnikova, N.S., Kurlov, A.S., Uritzkaya, A.A. et al. Diffraction analysis of nanocrystalline particle size of lead and cadmium sulfides prepared by chemical deposition from aqueous solutions. J Struct Chem 45 (Suppl 1), S154–S159 (2004). https://doi.org/10.1007/s10947-006-0111-4
Received:
Issue Date:
DOI: https://doi.org/10.1007/s10947-006-0111-4