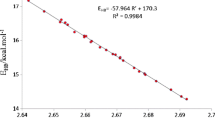
Abstract
Our previous study has revealed that para-substituents have opposite electronic effects on the C-S bond lengths of thiophenols and thiophenolic radicals. Although a theoretical elucidation has been given, it has not been supported by theoretically calculated atomic charges. To give an alternative explanation, we calculated the C-S bond lengths, C-S bond electron densities, and Mulliken charges on the carbon and sulfur atoms for thiophenols, thiophenolic radicals, and thiophenolic radical cations by means of the B3LYP density functional theory method using the 6-31G(d, p) basis set. It was revealed that the C-S bond length is adequately defined in terms of C-S bond electron density. The distinct electronic effects on the C-S bond lengths of thiophenols, thiophenolic radicals and thiophenolic radical cations are well elucidated by the different electronic states (electron-deficient or-rich) of the phenyl ring and SH group.
Similar content being viewed by others
References
G. Scott, Bull. Chem. Soc. Jpn., 61, 165–170 (1988).
B. Halliwell, R. Aeschbach, J. Loliger, and O. I. Aruoma, Food Chem. Toxic., 33, 601–617 (1995).
O. I. Aruoma, Free Radic. Biol. Med., 20, 675–705 (1996).
A. N. Alexidis, E. A. Rekka, V. J. Demopolos, and P. N. Kourounakis, J. Pharm. Pharmacol., 47, 131–137 (1995).
V. Zoete, F. Bailly, J.-P. Catteau, and J.-L. Bernier, J. Chem. Soc., Perkin Trans. 1, 2983–2988 (1997).
Y. Fu, B. L. Lin, K. S. Song, L. Liu, and Q. X. Guo, J. Chem. Soc., Perkin Trans. 2, 1223–1230 (2002).
M. J. S. Dewar, E. G. Zoebisch, E. F. Healy, and J. J. P. Stewart, J. Am. Chem. Soc., 107, 3902–3909 (1985).
M. J. Frisch, G. W. Trucks, H. B. Schlegel, et al., Gaussian-98, Revision A. 11, Gaussian, Inc., Pittsburgh, PA (1998).
K. R. S. Chandrakumar and S. Pal, Int. J. Mol. Sci., 3, 324–337 (2002).
H. D. Wells, W. N. Delgass, and K. T. Thomson, J. Chem. Phys., 117, 10597–10603 (2002).
R. Hermann, S. Naumov, G. R. Mahalaxmi, and O. Brede, Chem. Phys. Lett., 324, 265–272 (2000).
M. R. Ganapathi, R. Hermann, S. Naumov, and O. Brede, Phys. Chem. Chem. Phys., 2, 4947–4955 (2000).
R. Hermann, S. Naumov, and O. Brede, J. Mol. Struct. (Theochem), 532, 69–80 (2000).
O. Brede, S. Kapoor, T. Mukherjee, R. Hermann, and S. Naumov, Phys. Chem. Chem. Phys., 4, 5096–5104 (2002).
H.-Y. Zhang and H.-F. Ji, J. Mol. Struct. (Theochem)., 663, 167–174 (2003).
(a) The Brown parameter σ +p is a constant for substituents conjugated with the reaction center effectively delocalizing the positive charge [16b]. (b) C. Hansch, A. Leo, and R. W. Taft, Chem. Rev., 91, 165–195 (1991).
Author information
Authors and Affiliations
Additional information
Original Russian Text Copyright © 2005 by Hong-Fang Ji, Liang Shen, and Hong-Yu Zhang
__________
Translated from Zhurnal Strukturnoi Khimii, Vol. 46, No. 2, pp. 355–360, March–April, 2005.
Rights and permissions
About this article
Cite this article
Ji, HF., Shen, L. & Zhang, HY. Theoretical reinvestigation of opposite electronic effects on bond lengths in thiophenols and thiophenolic radicals. J Struct Chem 46, 347–351 (2005). https://doi.org/10.1007/s10947-006-0052-y
Received:
Issue Date:
DOI: https://doi.org/10.1007/s10947-006-0052-y