Abstract
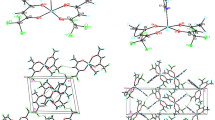
Synthesis and X-ray diffraction study of trans-bis-(2-(methylimino)-4-pentanonato)Cu(II), which is methyl-substituted ketoiminate, is reported. Crystal data for CuN2O2C12H20: a = 7.374(1) Å, b = 9.171(1) Å, c = 10.823(2) Å; α = 96.51(1)°, β = 106.12(1)°, γ = 96.81(1)°, space group P \(\bar 1\), Z = 2, d calc = 1.38 g/cm3, d exp = 1.37 g/cm3, R = 0.037. The structure is molecular and consists of isolated trans-complexes. The coordination polyhedron of the copper atom is intermediate between the square and tetrahedron; the average distances are Cu-O 1.91 Å and Cu-N 1.95 Å, the O-Cu-O and N-Cu-N trans bond angles are 145.5° and 150.3°, respectively. The O-Cu-N chelate angle is 94.6°. The calculated energies of van der Waals intermolecular interactions are compared with the thermogravimetric characteristics of the complex with ketoiminate and copper(II) ethylenediamine-bis-acetylacetonate.
Similar content being viewed by others
REFERENCES
Yu. V. Chumachenko, I. K. Igumenov, and S. V. Zemskov, Koordinats. Khim., 5, No.11, 1625–1627 (1979).
E. A. Mazurenko, O. A. Posilskii, V. Ya. Zub, et al., Ukr. Khim. Zh., 58, No.1, 16/17 (1992).
G. E. Gurr, Inorg. Chem., 3, No.4, 614/615 (1964).
P. A. Stabnikov, I. A. Baidina, S. V. Sysoev, et al., Zh. Strukt. Khim., 44, No.6, 1138–1145 (2003).
G. E. Gurr, Acta Crystallogr., 24B, 1511–1517 (1968).
A. Baidina, S. A. Gromilov, G. I. Zharkova, and P. A. Stabnikov, Zh. Strukt. Khim., 44, No.3, 488–497 (2003).
D. Hall, A. D. Rae, and T. N. Waters, J. Chem. Soc., 12, 5897–5901 (1963).
M. J. Hampden-Smith and T. T. Kodas, Chem. Commun., No. 3, 217–219 (1992).
H. F. Holtzclaw, J. P. Collman, and R. M. Alire, J. Am. Chem. Soc., 80, No.5, 1100–1103 (1958).
L. A. Kazitsina, N. B. Kupletskaya, and Yu. A. Kolesnik, Zh. Org. Khim., 31, No.1, 313–323 (1961).
R. H. Holm, G. W. Everett, and A. Chakravorty, Prog. Inorg. Chem., 7, 83–241 (1966).
K. Igumenov, Chemical Sciences Candidate’s Dissertation, Institute of Inorganic Chemistry, Novosibirsk (1973).
P. T. McCarthy, R. J. Hovey, U. Keihei, and A. E. Martell, J. Am. Chem. Soc., 77, No.2, 5820–5824 (1955).
G. M. Sheldrick, SHELX-97, Release 97-1, Univ. Göttingen (1997).
R. W. Moshier and R. E. Sievers, Gas Chromatography of Metal Chelates [Russian translation], Mir, Moscow (1967).
A. Baidina, Chemical Sciences Candidate’s Dissertation, Institute of Inorganic Chemistry, Novosibirsk (2000).
G. I. Zharkova, P. A. Stabnikov, V. M. Grankin, et al., Koordinats. Khim., 26, No.8, 614–620 (2000).
Author information
Authors and Affiliations
Additional information
Original Russian Text Copyright © 2004 by I. A. Baidina, P. A Stabnikov, A. D. Vasiliev, S. A. Gromilov, and I. K. Igumenov
Translated from Zhurnal Strukturnoi Khimii, Vol. 45, No. 4, pp. 706–712, July–August, 2004.
Rights and permissions
About this article
Cite this article
Baidina, I.A., Stabnikov, P.A., Vasiliev, A.D. et al. Crystal and molecular structure of copper(II) trans-bis-(2-(methylimino)-4-pentanonate). J Struct Chem 45, 671–677 (2004). https://doi.org/10.1007/s10947-005-0043-4
Received:
Issue Date:
DOI: https://doi.org/10.1007/s10947-005-0043-4