Abstract
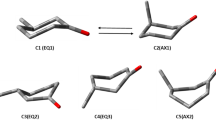
The molecular conformations of 2,6-bis-(4-phenyl)benzylidene-3R-methyl-cyclohexanone and its mono-(4-phenyl)benzylidene methylcyclohexanone isomers were investigated by molecular simulation using the semiempirical AM1 and PM3 methods and by analyzing the experimental spin-spin coupling constants in the PMR spectra. Mesomorphism and the twisting ability of the induced cholesteric mesophases of the title compounds are analyzed using the ratios between conformers with the axial and equatorial methyl groups and other peculiarities of the spatial structure of molecules (differences in anisometricity and in the degree of flattening of the cinnamoyl fragment). The equatorial orientation of the methyl group in the dominant conformations generally favors the formation of mesophases. The twisting ability is higher for chiral compounds with an axial methyl substituent and with the chiral center directly bonded to the enone and arylidene groups.
Similar content being viewed by others

REFERENCES
C. J. Both D. A. Dunmur J. W. Goodby et al. (1994) J. Mater. Chem. 4 IssueID5 747–759
A. Petrenko J. W. Goodby J. Meier (2000) Funct. Mater. 7 IssueID3 429–437
L. A. Kutulya (1998) ArticleTitleNonlinear optics of liquid and photorefractive crystals Proc. SPIE 3488 84–96
L. A. Kutulya (2001) Chiral Organic Compounds in Liquid Crystal Systems with Induced Helical Structures. Functional Materials for Science and Technology Institute of Single Crystals Kharkov 381–418
A. I. Krivoshey L. A. Kutulya N. S. Pivnenko et al. (2002) 9th Int. Conf. “Nonlinear Optics of Liquid and Photorefractive Crystals” Crimea Ukraine
D. Demus C. Krieg W. Weissflog (1982) Z. Chem. 22 446–447
J. Lub W. Ten Hoeve W. P. M. Nijssen et al. (2002) Liq. Cryst. 29 IssueID7 995–1000
V. V. Vashchenko, L. A. Kutulya, M. N. Pivnenko, and N. I. Shkolnikova, Izv. Akad. Nauk, Ser. Khim., No. 11, 2276–2288 (2003).
M. J. S. Dewar E. G. Zoebisch E. F. Healy J. J. P. Stewart (1985) J. Am. Chem. Soc. 107 IssueID13 3902–3909
J. J. P. Stewart (1989) J. Comp. Chem. 10 IssueID2 221–264
V. V. Vashchenko, N. S. Pivnenko, L. A. Kutulya et al., Izv. Akad. Nauk, Ser. Khim., No. 7, 1221–1233 (2000).
L. A. Kutulya N. S. Pivnenko A. S. Petrenko et al. (2001) Zh. Strukt. Khim. 42 IssueID1 101–110
N. S. Pivnenko T. G. Drushlyak L. A. Kutulya et al. (2002) Magn. Res. Chem. 40 IssueID9 566–572
N. S. Pivnenko A. I. Krivoshey L. A. Kutulya (2003) Magn. Res. Chem. 41 IssueID7 517–525
L. A. Kutulya G. P. Semenkova S. N. Yarmolenko et al. (1993) Kristallografiya 38 IssueID1 183–191
V. V. Vashchenko, “Stereochemistry of reactions of p-menthan-3-one enolates and their 2-arylidene derivatives with electrophilic reagents,” Chemical Sciences Candidate’s Dissertation, Institute of Single Crystals, Kharkov (1997).
C. A. G. Haasnoot F. A. A. M. Leeuw C. Altona (1980) Tetrahedron 36 IssueID19 2783–2792
N. S. Pivnenko, V. V. Vashchenko, L. A. Kutulya, et al., Izv. Ross. Akad. Nauk, Ser. Khim., No. 9, 1519–1527 (2001).
R. J. Abraham, D. S. Ribeiro, J. Chem. Soc., Perkin Trans. 2, 302–307 (2001).
K. L. Servis D. J. Bowler C. Ishii (1975) J. Am. Chem. Soc. 97 IssueID8 73–80
R. J. Abraham D. J. Chadwick L. Griffiths F. Sancassan (1980) J. Am. Chem. Soc. 102 IssueID15 5128–5130
D. S. Ribeiro R. J. Abraham (2002) Magn. Res. Chem. 40 IssueID1 49–56
D. S. Ribeiro P. R. Olivato R. Rittner (2002) Magn. Res. Chem. 38 IssueID8 627–638
E. V. Popova A. P. Fedoryako V. V. Vashchenko L. A. Kutulya (2000) Zh. Fiz. Khim. 74 IssueID11 1967–1971
L. A. Kutulya, “Chiral α,β-unsaturated carbonyl compounds in liquid crystal systems with induced helical structures,” Chemical Sciences’ Doctoral Dissertation, Kharkov (1992).
Author information
Authors and Affiliations
Additional information
Original Russian Text Copyright © 2004 by L. A. Kutulya, A. I. Krivoshei, N. S. Pivnenko, and N. I. Shkolnikova
Translated from Zhurnal Strukturnoi Khimii, Vol. 45, No. 3, pp. 419–429, May–June, 2004.
Rights and permissions
About this article
Cite this article
Kutulya, L.A., Krivoshei, A.I., Pivnenko, N.S. et al. Molecular conformation effects on mesomorphism and twisting ability of chiral cyclohexanones in mesophases. J Struct Chem 45, 395–404 (2004). https://doi.org/10.1007/s10947-005-0005-x
Received:
Issue Date:
DOI: https://doi.org/10.1007/s10947-005-0005-x