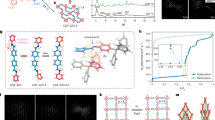
Abstract
In this study, a covalent-triazine framework (i.e., CTF-1) was synthesized through the ionothermal cyclotrimerization method utilizing 1,4-dicyanobenzene (DCB) as the monomer and molten ZnCl2 as both the solvent and Lewis acid catalyst at 400 ℃. The obtained microporous crystalline material was systematically characterized by employing Fourier-transform infrared (FT-IR) spectroscopy, powder X-ray diffraction (PXRD), N2 adsorption–desorption isotherms (BET-NLDFT surface area-pore size measurements), X-ray photoelectron spectroscopy (XPS), Raman spectroscopy, transmission electron microscopy (TEM), scanning electron microscopy (SEM), solid-state 13C NMR spectroscopy, elemental analysis (EA), and thermogravimetric analysis (TGA). The adsorption equilibrium and kinetic data for SO2, CO2, NO2, CH4, CO, NO, and N2 gases onto the surfaces of CTF-1 were volumetrically measured at 25 and 35 ℃ and gas pressures of up to 1 bar. To systematically realize the gas adsorption characteristics of CTF-1, various isotherm models, including the Langmuir, Sips, and Toth models, were employed to correlate the experimental adsorption isotherms. Besides, different kinetic models such as pseudo-first-order (PFO), pseudo-second-order (PSO), and fractal-like pseudo-first-order (FL-PFO) models were applied to analyze the experimental adsorption kinetic data. In the meantime, to further investigate the adsorption mechanism of gases upon the CTF-1 surface, density-functional theory (DFT) calculations were performed at ωB97XD level of theory.







Similar content being viewed by others
References
L. Jeffry, M.Y. Ong, S. Nomanbhay, M. Mofijur, M. Mubashir, P.L. Show, Greenhouse gases utilization: a review. Fuel 301, 121017 (2021). https://doi.org/10.1016/j.fuel.2021.121017
A. Kumar, A. Thanki, H. Padhiyar, N.K. Singh, S. Pandey, M. Yadav, Z.-G. Yu, Greenhouse gases emission control in WWTS via potential operational strategies: a critical review. Chemosphere 273, 129694 (2021). https://doi.org/10.1016/j.chemosphere.2021.129694
T. Cowan, S. Undorf, G.C. Hegerl, L.J. Harrington, F.E. Otto, Present-day greenhouse gases could cause more frequent and longer dust bowl heatwaves. Nat. Clim. Chang. 10(6), 505–510 (2020). https://doi.org/10.1038/s41558-020-0771-7
S. Pourebrahimi, M. Pirooz, S. Ahmadi, M. Kazemeini, L. Vafajoo, Nanoengineering of metal-based electrocatalysts for carbon dioxide (CO2) reduction: a critical review. Mater. Today Phys. (2023). https://doi.org/10.1016/j.mtphys.2023.101250
S. Pourebrahimi, M. Kazemeini, M. Zaroudi, H. Bozorgzadeh, Methane adsorption on carbonaceous microporous materials prepared from cellulose and lignin: equilibrium and kinetic studies. Sci. Iran. 25(6), 3368–3380 (2018). https://doi.org/10.24200/SCI.2018.5131.1114
M. Holmberg, A. Akujärvi, S. Anttila, I. Autio, M. Haakana, V. Junttila, N. Karvosenoja, P. Kortelainen, A. Mäkelä, K. Minkkinen, Sources and sinks of greenhouse gases in the landscape: approach for spatially explicit estimates. Sci. Total. Environ. 781, 146668 (2021). https://doi.org/10.1016/j.scitotenv.2021.146668
J. Deng, X. Wang, Z. Wei, L. Wang, C. Wang, Z. Chen, A review of NOx and SOx emission reduction technologies for marine diesel engines and the potential evaluation of liquefied natural gas fuelled vessels. Sci. Total. Environ. 766, 144319 (2021). https://doi.org/10.1016/j.scitotenv.2020.144319
D.J. Tao, F.F. Chen, Z.Q. Tian, K. Huang, S.M. Mahurin, D.E. Jiang, S. Dai, Highly efficient carbon monoxide capture by carbanion-functionalized ionic liquids through C-site interactions. Angew. Chem. 129(24), 6947–6951 (2017). https://doi.org/10.1002/ange.201701919
X. Zhang, B. Gao, A.E. Creamer, C. Cao, Y. Li, Adsorption of VOCs onto engineered carbon materials: a review. J. Hazard. Mater. 338, 102–123 (2017). https://doi.org/10.1016/j.jhazmat.2017.05.013
S. Pourebrahimi, Upcycling face mask wastes generated during COVID-19 into value-added engineering materials: a review. Sci. Total. Environ. 851, 158396 (2022). https://doi.org/10.1016/j.scitotenv.2022.158396
S. Pourebrahimi, M. Pirooz, Functionalized covalent triazine frameworks as promising platforms for environmental remediation: a review. Clean. Chem. Eng. 2, 100012 (2022). https://doi.org/10.1016/j.clce.2022.100012
G. Férey, C. Serre, T. Devic, G. Maurin, H. Jobic, P.L. Llewellyn, G. De Weireld, A. Vimont, M. Daturi, J.-S. Chang, Why hybrid porous solids capture greenhouse gases? Chem. Soc. Rev. 40(2), 550–562 (2011). https://doi.org/10.1039/C0CS00040J
X. Li, L. Zhang, Z. Yang, P. Wang, Y. Yan, J. Ran, Adsorption materials for volatile organic compounds (VOCs) and the key factors for VOCs adsorption process: a review. Sep. Purif. Technol. 235, 116213 (2020). https://doi.org/10.1016/j.seppur.2019.116213
G. Zhang, Y. Sun, P. Zhao, Y. Xu, A. Su, J. Qu, Characteristics of activated carbon modified with alkaline KMnO4 and its performance in catalytic reforming of greenhouse gases CO2/CH4. J. CO2 Util. 20, 129–140 (2017). https://doi.org/10.1016/j.jcou.2017.05.013
H. Rezvani, S. Fatemi, J. Tamnanloo, Activated carbon surface modification by catalytic chemical vapor deposition of natural gas for enhancing adsorption of greenhouse gases. J. Environ. Chem. Eng. 7(3), 103085 (2019). https://doi.org/10.1016/j.jece.2019.103085
M. Idrees, V. Rangari, S. Jeelani, Sustainable packaging waste-derived activated carbon for carbon dioxide capture. J. CO2 Util. 26, 380–387 (2018). https://doi.org/10.1016/j.jcou.2018.05.016
S. Kumar, R. Srivastava, J. Koh, Utilization of zeolites as CO2 capturing agents advances and future perspectives. J. CO2 Util. 41, 101251 (2020). https://doi.org/10.1016/j.jcou.2020.101251
S.M. Wilson, F.H. Tezel, Direct dry air capture of CO2 using VTSA with faujasite zeolites. Ind. Eng. Chem. Res. 59(18), 8783–8794 (2020). https://doi.org/10.1021/acs.iecr.9b04803
P. Murge, S. Dinda, S. Roy, Zeolite-based sorbent for CO2 capture: preparation and performance evaluation. Langmuir 35(46), 14751–14760 (2019). https://doi.org/10.1021/acs.langmuir.9b02259
T.K. Pal, D. De, P.K. Bharadwaj, Metal–organic frameworks for the chemical fixation of CO2 into cyclic carbonates. Coord. Chem. Rev. 408, 213173 (2020). https://doi.org/10.1016/j.ccr.2019.213173
S. Pourebrahimi, M. Kazemeini, L. Vafajoo, Embedding graphene nanoplates into MIL-101 (Cr) pores: synthesis, characterization, and CO2 adsorption studies. Ind. Eng. Chem. Res. 56(14), 3895–3904 (2017). https://doi.org/10.1021/acs.iecr.6b04538
S. Pourebrahimi, M. Kazemeini, E.G. Babakhani, A. Taheri, Removal of the CO2 from flue gas utilizing hybrid composite adsorbent MIL-53 (Al)/GNP metal-organic framework. Microporous Mesoporous Mater. 218, 144–152 (2015). https://doi.org/10.1016/j.micromeso.2015.07.013
J. Wang, S. Zhuang, Covalent organic frameworks (COFs) for environmental applications. Coord. Chem. Rev. 400, 213046 (2019). https://doi.org/10.1016/j.ccr.2019.213046
Y. Zeng, R. Zou, Y. Zhao, Covalent organic frameworks for CO2 capture. Adv. Mater. 28(15), 2855–2873 (2016). https://doi.org/10.1002/adma.201505004
J.L. Mendoza-Cortes, T.A. Pascal, W.A. Goddard III., Design of covalent organic frameworks for methane storage. J. Phys. Chem. A 115(47), 13852–13857 (2011). https://doi.org/10.1021/jp209541e
X. Sheng, H. Shi, L. Yang, P. Shao, K. Yu, X. Luo, Rationally designed conjugated microporous polymers for contaminants adsorption. Sci. Total. Environ. 750, 141683 (2021). https://doi.org/10.1016/j.scitotenv.2020.141683
S. Qiao, W. Huang, Z. Du, X. Chen, F.-K. Shieh, R. Yang, Phosphine oxide-based conjugated microporous polymers with excellent CO2 capture properties. New J. Chem. 39(1), 136–141 (2015). https://doi.org/10.1039/C4NJ01477D
H. Wang, D. Jiang, D. Huang, G. Zeng, P. Xu, C. Lai, M. Chen, M. Cheng, C. Zhang, Z. Wang, Covalent triazine frameworks for carbon dioxide capture. J. Mater. Chem. A 7(40), 22848–22870 (2019). https://doi.org/10.1039/C9TA06847C
K. Wang, Y. Tang, Q. Jiang, Y. Lan, H. Huang, D. Liu, C. Zhong, A thiophene-containing covalent triazine-based framework with ultramicropore for CO2 capture. J. Energy Chem. 26(5), 902–908 (2017). https://doi.org/10.1016/j.jechem.2017.07.007
A. Mukhtar, N.B. Mellon, M.A. Bustam, S. Saqib, S.-P. Lee, F.A. Kareem, S. Ullah, Impact of amine functionality on the selective CO2/CH4 adsorption behavior of porous covalent triazine adsorbent. J. Nat. Gas Sci. Eng. 83, 103582 (2020). https://doi.org/10.1016/j.jngse.2020.103582
Y. Zhang, S. Jin, Recent advancements in the synthesis of covalent triazine frameworks for energy and environmental applications. Polymers 11(1), 31 (2018). https://doi.org/10.3390/polym11010031
S. Pourebrahimi, M. Pirooz, Reversible iodine vapor capture using bipyridine-based covalent triazine framework: experimental and computational investigations. Chem. Eng. J. Adv. 8, 100150 (2021). https://doi.org/10.1016/j.ceja.2021.100150
M. Liu, L. Guo, S. Jin, B. Tan, Covalent triazine frameworks: synthesis and applications. J. Materi. Chem. A 7(10), 5153–5172 (2019). https://doi.org/10.1039/C8TA12442F
C. Krishnaraj, H.S. Jena, K. Leus, P. Van Der Voort, Covalent triazine frameworks–a sustainable perspective. Green Chem. 22(4), 1038–1071 (2020). https://doi.org/10.1039/C9GC03482J
S. Pourebrahimi, M. Pirooz, A. De Visscher, G.H. Peslherbe, Highly efficient and reversible iodine capture utilizing amorphous conjugated covalent triazine-based porous polymers: Experimental and computational studies. J. Environ. Chem. Eng. 10(3), 107805 (2022). https://doi.org/10.1016/j.jece.2022.107805
P. Kuhn, M. Antonietti, A. Thomas, Porous, covalent triazine-based frameworks prepared by ionothermal synthesis. Angew. Chem. Int. Ed. 47(18), 3450–3453 (2008). https://doi.org/10.1002/anie.200705710
S. Kuecken, J. Schmidt, L. Zhi, A. Thomas, Conversion of amorphous polymer networks to covalent organic frameworks under ionothermal conditions: a facile synthesis route for covalent triazine frameworks. J. Mater. Chem. A 3(48), 24422–24427 (2015). https://doi.org/10.1039/C5TA07408H
S. Ren, M.J. Bojdys, R. Dawson, A. Laybourn, Y.Z. Khimyak, D.J. Adams, A.I. Cooper, Porous, fluorescent, covalent triazine-based frameworks via room-temperature and microwave-assisted synthesis. Adv. Mater. 24(17), 2357–2361 (2012). https://doi.org/10.1002/adma.201200751
P. Puthiaraj, S.-S. Kim, W.-S. Ahn, Covalent triazine polymers using a cyanuric chloride precursor via Friedel-Crafts reaction for CO2 adsorption/separation. Chem. Eng. J. 283, 184–192 (2016). https://doi.org/10.1016/j.cej.2015.07.069
S.N. Talapaneni, T.H. Hwang, S.H. Je, O. Buyukcakir, J.W. Choi, A. Coskun, Elemental-sulfur-mediated facile synthesis of a covalent triazine framework for high-performance lithium–sulfur batteries. Angew. Chem. Int. Ed. 55(9), 3106–3111 (2016). https://doi.org/10.1002/anie.201511553
T. Zhou, Y. Zhao, J.W. Choi, A. Coskun, Lithium-salt mediated synthesis of a covalent triazine framework for highly stable lithium metal batteries. Angew. Chem. 131(47), 16951–16955 (2019). https://doi.org/10.1002/ange.201908513
R. Gomes, P. Bhanja, A. Bhaumik, A triazine-based covalent organic polymer for efficient CO2 adsorption. Chem. Commun. 51(49), 10050–10053 (2015). https://doi.org/10.1039/C5CC02147B
T. Lu, F. Chen, Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 33(5), 580–592 (2012). https://doi.org/10.1002/jcc.22885
D.Y. Osadchii, A.I. Olivos-Suarez, A.V. Bavykina, J. Gascon, Revisiting nitrogen species in covalent triazine frameworks. Langmuir 33(50), 14278–14285 (2017). https://doi.org/10.1021/acs.langmuir.7b02929
M.J. Bojdys, J. Jeromenok, A. Thomas, M. Antonietti, Rational extension of the family of layered, covalent, triazine-based frameworks with regular porosity. Adv. Mater. 22(19), 2202–2205 (2010). https://doi.org/10.1002/adma.200903436
M. Thommes, K. Kaneko, A.V. Neimark, J.P. Olivier, F. Rodriguez-Reinoso, J. Rouquerol, K.S. Sing, Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 87(9–10), 1051–1069 (2015). https://doi.org/10.1515/pac-2014-1117
S. Pourebrahimi, M. Kazemeini, A kinetic study of facile fabrication of MIL-101 (Cr) metal-organic framework: effect of synthetic method. Inorg. Chim. Acta 471, 513–520 (2018). https://doi.org/10.1016/j.ica.2017.11.033
S. Pourebrahimi, M. Pirooz, Synthesis of a novel freestanding conjugated triazine-based microporous membrane through superacid-catalyzed polymerization for superior CO2 separation. Chem. Eng. J. Adv. 11, 100315 (2022). https://doi.org/10.1016/j.ceja.2022.100315
C. Yin, Z. Zhang, J. Zhou, Y. Wang, Single-layered nanosheets of covalent triazine frameworks (CTFs) by mild oxidation for molecular-sieving membranes. ACS Appl. Mater. Interfaces 12(16), 18944–18951 (2020). https://doi.org/10.1021/acsami.0c03246
G. Tuci, M. Pilaski, H. Ba, A. Rossin, L. Luconi, S. Caporali, C. Pham-Huu, R. Palkovits, G. Giambastiani, Unraveling surface basicity and bulk morphology relationship on covalent triazine frameworks with unique catalytic and gas adsorption properties. Adv. Func. Mater. 27(7), 1605672 (2017). https://doi.org/10.1002/adfm.201605672
Y. Fu, Z. Wang, S. Li, X. He, C. Pan, J. Yan, G. Yu, Functionalized covalent triazine frameworks for effective CO2 and SO2 removal. ACS Appl. Mater. Interfaces 10(42), 36002–36009 (2018). https://doi.org/10.1021/acsami.8b13417
H. Zhu, W. Lin, Q. Li, Y. Hu, S. Guo, C. Wang, F. Yan, Bipyridinium-based ionic covalent triazine frameworks for CO2, SO2, and NO capture. ACS Appl. Mater. Interfaces 12(7), 8614–8621 (2020). https://doi.org/10.1021/acsami.9b15903
J. Shen, A. Dailly, M. Beckner, Natural gas sorption evaluation on microporous materials. Microporous Mesoporous Mater. 235, 170–177 (2016). https://doi.org/10.1016/j.micromeso.2016.08.013
E.D. Revellame, D.L. Fortela, W. Sharp, R. Hernandez, M.E. Zappi, Adsorption kinetic modeling using pseudo-first order and pseudo-second order rate laws: a review. Clean. Eng. Technol. 1, 100032 (2020). https://doi.org/10.1016/j.clet.2020.100032
L. Largitte, R. Pasquier, A review of the kinetics adsorption models and their application to the adsorption of lead by an activated carbon. Chem. Eng. Res. Des. 109, 495–504 (2016). https://doi.org/10.1016/j.cherd.2016.02.006
Author information
Authors and Affiliations
Contributions
SP: conceptualization, methodology, supervision, writing the first draft, review and editing the final draft MP: investigation, resources, methodology MK: supervision, review and editing the final draft LV: supervision, review and editing the final draft.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pourebrahimi, S., Pirooz, M., Kazemeini, M. et al. Synthesis, characterization, and gas (SO2, CO2, NO2, CH4, CO, NO, and N2) adsorption properties of the CTF-1 covalent triazine framework-based porous polymer: experimental and DFT studies. J Porous Mater 31, 643–657 (2024). https://doi.org/10.1007/s10934-023-01538-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-023-01538-9