Abstract
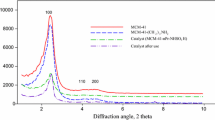
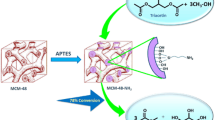
Herein an able, facile, coherent, and environmentally benign synthesis of the C–O coupling reaction based on the functionalized mesoporous silica SBA-16 (CoII immobilized on mesoporous SBA-16 functionalized by aminated 3-glycidyloxypropyltrimethoxysilane with aminoguanidine nitrate), is described. The mesostructured nanocatalyst (SBA-16/GPTMS-NH2–CoII) is characterized by various analytical techniques such as FT-IR, small-angle XRD, XRD, BET, TEM, FE-SEM, EDX, EDX-mapping, and ICP-OES analysis. Cage-like mesoporous SBA-16 efficiently managed dispersed the metal and organic species in the well-ordered siliceous frameworks. It was found that the aforementioned mesostructured catalyst with a special structure and particle size distribution (3–8 nm) displayed great catalytic performance to promote of the C–O cross-coupling reaction from the iodobenzene and phenol in solvent-free conditions. Plus, SBA-16/GPTMS-NH2–CoII as an inimitable heterogeneous nanocatalyst can be readily separated from the reaction mixture by simple filtration under reaction conditions and recycled at least five times without any loss of its catalytic efficiency.














Similar content being viewed by others
Data availability
All data supporting the findings of this study are available with the article, as well as the Supplementary Information file, or available from the corresponding authors upon reasonable request. Source data are provided in this paper.
References
J.-P. Corbet, G. Mignani, Selected patented cross-coupling reaction technologies. Chem. Rev. 106, 2651–2710 (2006)
M. Mondal, S.K. Bharadwaj, U. Bora, O-arylation with nitroarenes: metal-catalyzed and metal-free methodologies. New J. Chem. 39, 31–37 (2014)
F. Monnier, M. Taillefer, Catalytic C–C, C–N, and C–O Ullmann-type coupling reactions. Angew. Chem. Int. Ed. 48, 6954–6971 (2009)
C.-W. Qian, W.-L. Lv, Q.-S. Zong, M.-Y. Wang, D. Fang, Copper-catalyzed Ullmann-type synthesis of diaryl ethers assisted by salicylaldimine ligands. Chin. Chem. Lett. 25, 337–340 (2014)
C. Sambiagio, S.P. Marsden, A.J. Blacker, P.C. McGowan, Copper catalysed Ullmann type chemistry: from mechanistic aspects to modern development. Chem. Soc. Rev. 43, 3525–3550 (2014)
D.L. Boger, M.A. Patane, J. Zhou, Total synthesis of bouvardin, O-methylbouvardin, and O-methyl-N9-desmethylbouvardin. J. Am. Chem. Soc. 116, 8544–8556 (1994)
D. Kikelj, Recent progress in diaryl ether synthesis. Synthesis 2006, 2271–2285 (2006)
F.M. Moghaddam, M. Eslami, Immobilized palladium nanoparticles on MNPs@ A-N-AEB as an efficient catalyst for C-O bond formation in water as a green solvent. Appl. Organomet. Chem. 32, e4463 (2018)
P.M. MacQueen, J.P. Tassone, C. Diaz, M. Stradiotto, Exploiting ancillary ligation to enable nickel-catalyzed C-O cross-couplings of aryl electrophiles with aliphatic alcohols. J. Am. Chem. Soc. 140, 5023–5027 (2018)
H. Sun, Y. Sun, X. Tian, Y. Zhao, X. Qi, Nanosized ferric hydroxide catalyzed CO cross-coupling of phenol and halides to generate phenoxy ether. Asian J. Chem. 25, 6189–6191 (2013)
M.S. Hofmayer, A. Sunagatullina, D. Brösamlen, P. Mauker, P. Knochel, Stereoselective cobalt-catalyzed cross-coupling reactions of arylzinc chlorides with α-bromolactones and related derivatives. Org. Lett. 22, 1286–1289 (2020)
A. Mohammadinezhad, B. Akhlaghinia, Fe3O4@ boehmite-NH2-Co II NPs: an inexpensive and highly efficient heterogeneous magnetic nanocatalyst for the Suzuki-Miyaura and Heck-Mizoroki cross-coupling reactions. Green Chem. 19, 5625–5641 (2017)
P. Qiu, B. Ma, C.-T. Hung, W. Li, D. Zhao, Spherical mesoporous materials from single to multilevel architectures. Acc. Chem. Res. 52, 2928–2938 (2019)
H. Yang, X. Zhang, S. Li, X. Wang, J. Ma, The high catalytic activity and reusability of the proline functionalized cage-like mesoporous material SBA-16 for the asymmetric aldol reaction proceeding in methanol–water mixed solvent. RSC Adv. 4, 9292–9299 (2014)
Z. Wang, L. Wang, P. Li, Silica-anchored proline-copper (I) as an efficient and recyclable catalyst for the Sonogashira reaction. Synthesis 2008, 1367–1372 (2008)
F. Zhang, J. Yin, W. Chai, H. Li, Self-assembly of palladium nanoparticles on periodic mesoporous organosilica using an in situ reduction approach: catalysts for Ullmann reactions in water. Chemsuschem 3, 724–727 (2010)
P.-H. Liao, H.-M. Yang, Preparation of catalyst Ni–Cu/CNTs by chemical reduction with formaldehyde for steam reforming of methanol. Catal. Lett. 121, 274–282 (2008)
S.S. Ghodsinia, B. Akhlaghinia, Cu I anchored onto mesoporous SBA-16 functionalized by aminated 3-glycidyloxypropyltrimethoxysilane with thiosemicarbazide (SBA-16/GPTMS-TSC-Cu I): a heterogeneous mesostructured catalyst for S-arylation reaction under solvent-free conditions. Green Chem. 21, 3029–3049 (2019)
Z. Ma, H. Yang, Y. Qin, Y. Hao, G. Li, Palladium nanoparticles confined in the nanocages of SBA-16: enhanced recyclability for the aerobic oxidation of alcohols in water. J. Mol. Catal. A 331, 78–85 (2010)
A. Vinu, T. Mori, K. Ariga, New families of mesoporous materials. Sci. Technol. Adv. Mater. 7, 753–771 (2006)
D. Zhao, Q. Huo, J. Feng, B. Chmelka, G. Stucky, Tri-, tetra-, and octablock copolymer and nonionic surfactant syntheses of highly ordered, hydrothermally stable, mesoporous silica structures. J. Am. Chem. Soc. 120, 6024–6036 (1998)
T.-W. Kim et al., Tailoring the pore structure of SBA-16 silica molecular sieve through the use of copolymer blends and control of synthesis temperature and time. J. Phys. Chem. B 108, 11480–11489 (2004)
H. Yang et al., Asymmetric reactions on chiral catalysts entrapped within a mesoporous cage. Chem. Commun. (2007). https://doi.org/10.1039/b614635j
H. Yang, L. Zhang, W. Su, Q. Yang, C. Li, Asymmetric ring-opening of epoxides on chiral Co (Salen) catalyst synthesized in SBA-16 through the “ship in a bottle” strategy. J. Catal. 248, 204–212 (2007)
H. Yang, L. Zhang, L. Zhong, Q. Yang, C. Li, Enhanced cooperative activation effect in the hydrolytic kinetic resolution of epoxides on [Co (salen)] catalysts confined in nanocages. Angew. Chem. 119, 6985–6989 (2007)
R. Azevedo, R. Sousa, W. Macedo, E. Sousa, Combining mesoporous silica–magnetite and thermally-sensitive polymers for applications in hyperthermia. J. Sol-Gel. Sci. Technol. 72, 208–218 (2014)
F. Azimov, I. Markova, V. Stefanova, K. Sharipov, Synthesis and characterization of SBA-15 and Ti-SBA-15 nanoporous materials for DME catalysts. J. Univ. Chem. Technol. Metall. 47, 333–340 (2012)
F. Bérubé, S. Kaliaguine, Calcination and thermal degradation mechanisms of triblock copolymer template in SBA-15 materials. Microporous Mesoporous Mater. 115, 469–479 (2008)
N. Koshy, D. Singh, Fly ash zeolites for water treatment applications. J. Environ. Chem. Eng. 4, 1460–1472 (2016)
S. Sadjadi, Palladium nanoparticles immobilized on cyclodextrin-decorated halloysite nanotubes: efficient heterogeneous catalyst for promoting copper-and ligand-free Sonogashira reaction in water–ethanol mixture. Appl. Organomet. Chem. 32, e4211 (2018)
A.A. Alqadami, M. Naushad, Z.A. Alothman, A.A. Ghfar, Novel metal–organic framework (MOF) based composite material for the sequestration of U (VI) and Th (IV) metal ions from aqueous environment. ACS Appl. Mater. Interfaces 9, 36026–36037 (2017)
Z. Çelik, M. Gülfen, A.O. Aydın, Synthesis of a novel dithiooxamide–formaldehyde resin and its application to the adsorption and separation of silver ions. J. Hazard. Mater. 174, 556–562 (2010)
Q. Yao, Z.H. Lu, K. Yang, X. Chen, M. Zhu, Sci. Rep. 5, 15186 (2015)
M. Bagherzadeh, H. Mahmoudi, M. Amini, S. Gautam, K.H. Chae, Sci. Iran. 25, 1335–1343 (2018)
J. Balamurugan, R. Thangamuthu, A. Pandurangan, RSC Adv. 3, 4321–4331 (2013)
K.S. Sing, Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (recommendations 1984). Pure Appl. Chem. 57, 603–619 (1985)
F. Kleitz, L.A. Solovyov, G.M. Anilkumar, S.H. Choi, R. Ryoo, Transformation of highly ordered large pore silica mesophases (Fm 3 m, Im 3 m and p 6 mm) in a ternary triblock copolymer–butanol–water system. Chem. Commun. (2004). https://doi.org/10.1039/B403903C
W. Affo et al., Cobalt-catalyzed trimethylsilylmethylmagnesium-promoted radical alkenylation of alkyl halides: a complement to the Heck reaction. J. Am. Chem. Soc. 128, 8068–8077 (2006)
S. Maity, P. Dolui, R. Kancherla, D. Maiti, Introducing unactivated acyclic internal aliphatic olefins into a cobalt catalyzed allylic selective dehydrogenative Heck reaction. Chem. Sci. 8, 5181–5185 (2017)
M.E. Weiss, L.M. Kreis, A. Lauber, E.M. Carreira, Cobalt-catalyzed coupling of alkyl iodides with alkenes: deprotonation of hydridocobalt enables turnover. Angew. Chem. Int. Ed. 50, 11125–11128 (2011)
G.P. Cerai, B. Morandi, Atom-economical cobalt-catalysed regioselective coupling of epoxides and aziridines with alkenes. Chem. Commun. 52, 9769–9772 (2016)
K.M. Nicholas, Chemistry and synthetic utility of cobalt-complexed propargyl cations. Acc. Chem. Res. 20, 207–214 (1987)
R.K. Sharma et al., Maghemite-copper nanocomposites: applications for ligand-free cross-coupling (C− O, C− S, and C− N) reactions. ChemCatChem 7, 3495–3502 (2015)
K. Swapna, S.N. Murthy, M.T. Jyothi, Y.V.D. Nageswar, Recyclable heterogeneous copper oxide on alumina catalyzed coupling of phenols and alcohols with aryl halides under ligand-free conditions. Org. Biomol. Chem. 9, 5978–5988 (2011)
R. Ghorbani-Vaghei, S. Hemmati, H. Veisi, An in situ generated CuI/metformin complex as a novel and efficient catalyst for C-N and C–O cross-coupling reactions. Tetrahedron Lett. 54, 7095–7099 (2013)
A. Dhakshinamoorthy, A.M. Asiri, H. Garcia, Metal–organic frameworks catalyzed C-C and C–heteroatom coupling reactions. Chem. Soc. Rev. 44, 1922–1947 (2015)
A. Majumder, R. Gupta, M. Mandal, M. Babu, D. Chakraborty, Air-stable palladium (0) phosphine sulfide catalysts for Ullmann-type C-N and C–O coupling reactions. J. Organomet. Chem. 781, 23–34 (2015)
M. Hosseini-Sarvari, Z. Razmi, Highly active recyclable heterogeneous Pd/ZnO nanoparticle catalyst: Sustainable developments for the C-O and C–N bond cross-coupling reactions of aryl halides under ligand-free conditions. RSC Adv. 4, 44105–44116 (2014)
P. Zhang et al., Mesoporous nitrogen-doped carbon for copper-mediated Ullmann-type C-O/–N/–S cross-coupling reactions. RSC Adv. 3, 1890–1895 (2013)
R. Hayes, C. Allen, Journal of inorganic and organometallic polymers and materials 20: polymerization of cyclophosphazenes with spriocyclic methacrylate containing substituents. Cell. Polym. 30, 344–345 (2011)
Funding
The author is grateful for the partial support of this study by Qeshm Branch, Islamic Azad University.
Author information
Authors and Affiliations
Contributions
Iman khosravi co-wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Eftekhar, M., Khosravi, I. CoII immobilized on aminated mesoporous SBA-16 (SBA-16/GPTMS-NH2–CoII): a highly efficient mesostructured catalyst for the C–O bond formation under solvent-free conditions. J Porous Mater 30, 1255–1272 (2023). https://doi.org/10.1007/s10934-022-01414-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-022-01414-y