Abstract
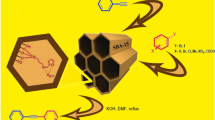
In this paper, we investigate the catalytic activity of CuI NPs supported on porous cross-linked poly(ethyleneamine)–polysulfonamide (PEA–PSA@CuI) as an effective and recyclable nanocatalyst for the synthesis of indolizine derivatives. Three-component reaction of 2-pyridine carbaldehyde with phenylacetylene and second-order amines in the presence of PEA–PSA@CuI as catalyst under solvent-free conditions resulted amino indolizine products in good to excellent yields. The present catalytic system offers advantages such as high catalytic activity in short reaction time, recovered the catalyst by centrifugation and recycled for five times without significant loss in its efficiency.






Similar content being viewed by others
References
J. Gerencsér, G. Dormán, F. Darvas, QSAR Comb. Sci. 25, 439–448 (2006)
D.J. Ramón, M. Yus, Angew. Chem. Int. Ed. 44, 1602–1634 (2005)
C. Hulme, V. Gore, Curr. Med. Chem. 10, 51–80 (2003)
R.J. Molyneux, L.F. James, Science 216, 190–191 (1982)
L.L. Gundersen, C. Charnock, A.H. Negussie, F. Rise, S. Teklu, Eur. J. Pharm. Sci. 30, 26–35 (2007)
L.L. Gundersen, K.E. Malterud, A.H. Negussie, F. Rise, S. Teklu, O.B. Østby, Bioorg. Med. Chem. 11, 5409–5419 (2003)
V.R. Vemula, S. Vurukonda, C.K. Bairi, Int. J. Pharm. Sci. Rev. Res. 11, 159–163 (2011)
T. Weide, L. Arve, H. Prinz, H. Waldmann, H. Kessler. Bioorg. Med. Chem. Lett 16, 59–63 (2006)
W.O. Foye, Foye’s Principles of Medicinal Chemistry (Lippincott Williams and Wilkins, 2008)
Y.D.A. James, K. Koya, H. Li, G. Liang, Z. Xia, W. Ying, L. Sun, Bioorg. Med. Chem. Lett. 18, 1784–1787 (2008)
M.W. Edwards, J.W. Daly, C.W. Myers, J. Nat. prod. 51, 1188–1197 (1988)
Y. Hu, J. Ren, L. Wang, X. Zhao, M. Zhang, K. Shimizu, C. Zhang, Phytochemistry 149, 12–23 (2018)
C. Sandeep, K.N. Venugopala, R.M. Gleiser, A. Chetram, B. Padmashali, R.S. Kulkarni, B. Odhav, Chem. Biol. Drug. Des. 88, 899–904 (2016)
N. Ortega, D.T.D Tang, S. Urban, D. Zhao, F. Glorius. Angew. Chem. Int. Ed. 52, 9500–9503 (2013).
C. Sandeep, B. Padmashali, R.S. Kulkarni, Tetrahedron Lett. 54, 6411–6414 (2013)
K.M. Elattar, I. Youssef, A.A. Fadda, Synth. Commun. 46, 719–744 (2016)
S.H. Hedges, R.B. Herbert, E. Knagg, V. Pasupathyt, Tetrahedron lett. 29, 807–810 (1988)
R.J. Andersen, D.J. Faulkner, C.H. He, G.D. Van Duyne, J. Clardy, J. Am. Chem. Soc. 107, 5492–5495 (1985)
V. Boekelheide, R.J. Windgassen, J. Am. Chem. Soc. 81, 1456–1459 (1959)
J. Hurst, T. Melton, D.G. Wibberley, J. Chem. Soc. 529, 2948–2955 (1965)
G. Poissonnet, M.H. Théret-Bettiol, R.H. Dodd, J. Org. Chem. 61, 2273–2282 (1996)
J. Barluenga, G. Lonzi, L. Riesgo, L.A. Lopez, M.J. Tomas, Am. Chem. Soc. 132, 13200–13202 (2010)
J.B. Xia, S.L. You, Org. Lett. 11, 1187–1190 (2009)
J. Kaloko, A. Hayford, Org. Lett. 7, 4305–4308 (2005)
P.L. Reddy, R. Arundhathi, M. Tripathi, D.S. Rawat, RSC Adv. 6, 53596–53601 (2016)
U.C. Rajesh, G. Purohit, D.S. Rawat, ACS. Sus. Chem. Eng. 3, 2397–2404 (2015)
E. Akhavan Taheri, S. Hemmati, M. Hekmati, H. Veisi, New J. Chem. 42, 14009–14009 (2018)
H. Veisi, M. Hamelian, S. Hemmati, A. Dalvand, Tetrahedron Lett. 58, 4440–4446 (2017)
H. Veisi, Y. Metghalchi, M. Hekmati, S. Samadzadeh, Appl. Organomet. Chem. 31, e3676 (2017)
M. Adib, V. Sadeghi, H. Veisi, Tetrahedron lett. 59, 1928–1931 (2018)
H. Hojat Veisi, S.A. Ahmadian, K. Mirshokraie, M.M. Didehban, Appl Zangeneh, Organomet. Chem. 33, e4737 (2019)
Y. Yang, C. Xie, Y. Xie, Y. Zhang, Org. Lett. 14, 957–959 (2012)
V. Helan, A.V. Gulevich, V. Gevorgyan, Chem. Sci. 6, 1928–1931 (2015)
S.S. Patil, S.V. Patil, V.D. Bobade, Synlett 2011, 1157–1159 (2011)
B. Sarmah, B. Satpati, R. Srivastava, RSC Adv. 6, 87066–87081 (2016)
G.H. Dang, H.Q. Lam, A.T. Nguyen, D.T. Le, T. Truong, N.T. Phan, J. Catal. 337, 167–176 (2016)
M.J. Albaladejo, F. Alonso, M.J. González-Soria, ACS Catalysis. 5, 3446–3456 (2015)
B. Yan, Y. Liu, Org. Lett. 9, 4323–4326 (2007)
S. Mishra, B. Naskar, R. Ghosh, Tetrahedron Lett. 53, 5483–5487 (2012)
S. Mishra, A.K. Bagdi, M. Ghosh, S. Sinha, A. Hajra, RSC Adv. 4, 6672–6676 (2014)
R. Ghorbani-Vaghei, H. Jalili, Synthesis 7, 1099–1102 (2005)
R. Ghorbani-Vaghei, N. Sarmast, Can. J. Chem. 95, 1073–1080 (2017)
S. Alavinia, R. Ghorbani-Vaghei, J. Rakhtshah, J. Yousefi Seyf, I. Ali Arabian, Appl Organomet Chem. 34(3), e5449 (2020)
R. Ghorbani-Vaghei, S. Alavinia, N. Sarmast, Appl. Organomet. Chem. 32, e 4038 (2018)
R. Ghorbani-Vaghei, S. Alavinia, Z. Merati, V. Izadkhah, Appl. Organomet. Chem. 32, e 4127 (2018)
A. Kharazmi, R. Ghorbani-Vaghei, S. Alavinia, ChemistrySelect. 5, 1424–1430 (2020)
A. Fatehi, R. Ghorbani-Vaghei, S. Alavinia, J. Mahmoodi, ChemistrySelect. 5, 944–951 (2020)
S. Alavinia, R. Ghorbani-Vaghei, New. J. Chem. 44, 13062–13073 (2020)
S. Alavinia, R. Ghorbani-Vaghei, J. Phys. Chem. Solids. 146, 109573 (2020)
F. Hamidi Dastjerdi, R. Ghorbani-Vaghei, S. Alavinia, Catal. Lett. (2020). https://doi.org/10.1007/s10562-020-03265-1
Acknowledgements
The authors wish to thank Bu-Ali Sina University, Center of Excellence Developmental of Environmentally Friendly Methods for Chemical Synthesis (CEDEFMCS) for financial support to carry out this research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Solgi, S., Ghorbani-Vaghei, R. & Alavinia, S. Application of copper iodide nanoparticles immobolized porous polysulfonamide gel as an effective nanocatalyst for synthesis of aminoindolizines. J Porous Mater 28, 289–298 (2021). https://doi.org/10.1007/s10934-020-00989-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-020-00989-8