Abstract
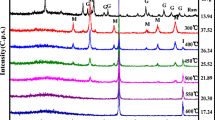
The application of nanoscale zero-valent iron (NZVI) in a promising remediation strategy was challenged by easy aggregation and surface passivation. To enhance their performance of Cr(VI) removal, layered double hydroxides (LDHs) supported NZVI (NZVI@LDH) with different loading amount via an in-situ way was successfully synthesized. The structural characterization and morphology revealed LDHs could stabilize and uniformly disperse NZVI on the surface of LDHs layer, however, superabundant NZVI still leaded to a visibly aggregation. Cr(VI) removal by NZVI@LDH was higher than that of the sum of NZVI and LDHs, revealing a synergistic effect within this system. The results of Cr(VI) removal displayed with the growth of iron loading amount, the removal efficiencies were raised firstly but declined afterwards. Kinetics studies for all samples showed Cr(VI) removal well fitted pseudo-second-order model and isotherm data was well described by Langmuir model. The effects of initial concentration and pH on Cr(VI) removal were also examined. Cr(VI) in the wastewater was reduced by NZVI to Cr(III), NZVI was oxidized into Fe(II) and Fe(III). The excellent removal performance suggested a promising strategy in sewage water remediation.





Similar content being viewed by others
References
Y. Liu, Z. Zhang, X. Sun et al., Design of three-dimensional macroporous reduced graphene oxide–Fe3O4 nanocomposites for the removal of Cr(VI) from wastewater. J. Porous Mater. 26, 109–119 (2019)
W. Tang, Y. Wu, A. Xu et al., Hybrid porous hypercrosslinking polyanilines: facile Friedel-Crafts preparation, CO2 capture and Cr(VI) removal properties. J. Porous Mater. 26, 1495–1505 (2019)
G. Li, X. Cui, S. Tang, Removal of Cr(VI) using mesoporous alumina with different kinds of pore structures. J. Porous Mater. 23, 919–926 (2016)
M. Goswami, L. Borah, D. Mahanta et al., Equilibrium modeling, kinetic and thermodynamic studies on the adsorption of Cr(VI) using activated carbon derived from matured tea leaves. J. Porous Mater. 21, 1025–1034 (2014)
L. Borah, K.K. Senapati, C. Borgohain et al., Preparation of ordered porous carbon from tea by chemical activation and its use in Cr(VI) adsorption. J. Porous Mater. 19, 767–774 (2012)
W. Sun, L. Chen, Y. Sun et al., The functional polypyrrole composite: a class of high-performing Cr(VI) ion adsorbents. J. Porous Mater. 24, 519–530 (2017)
Y.Q. Xing, X.M. Chen, D.H. Wang, Electrically regenerated ion exchange for removal and recovery of Cr(VI) from wastewater. Environ. Sci. Technol. 41(4), 1439–1443 (2007)
E. Molina-Jijón, G. Zarco-Márquez, O.N. Medina-Campos et al., Deferoxamine pretreatment prevents Cr(VI)-induced nephrotoxicity and oxidant stress: role of Cr(VI) chelation. Toxicology 291(1–3), 93–101 (2012)
J. Yoon, G. Amy, Y. Yoon, Transport of target anions, chromate (Cr (VI)), arsenate (As (V)), and perchlorate (ClO4-), through RO, NF, and UF membranes. Water Sci. Technol. 51(6–7), 327 (2005)
T. Wang, Y. Liu, J. Wang, In-situ remediation of hexavalent chromium contaminated groundwater and saturated soil using stabilized iron sulfide nanoparticles. J. Environ. Manag. 231, 679–686 (2019)
K. Selvi, S. Pattabhi, K. Kadirvelu, Removal of Cr(VI) from aqueous solution by adsorption onto activated carbon. Bioresour. Technol. 80(1), 87–89 (2001)
D. Jiang, G. Zeng, D. Huang et al., Remediation of contaminated soils by enhanced nanoscale zero valent iron. Environ. Res. 163, 217–227 (2018)
H. Lu, W. Wang, Z. Xiao et al., Facile synthesis of MCM-41/nano zero-valent iron composite for catalytic reduction of p-nitrophenol. J. Porous Mater. 22, 1559–1565 (2015)
Z. Shi, W. Shen, K. Yang et al., Hexavalent chromium removal by a new composite system of dissimilatory iron reduction bacteria Aeromonas hydrophila and nanoscale zero-valent iron. Chem. Eng. J. 362, 63–70 (2019)
Z. Chen, T. Wang, X. Jin et al., Multifunctional kaolinite-supported nanoscale zero-valent iron used for the adsorption and degradation of crystal violet in aqueous solution. J. Colloid Interface Sci. 398, 59–66 (2013)
H. Dong, L. Li, Y. Lu et al., Integration of nanoscale zero-valent iron and functional anaerobic bacteria for groundwater remediation: a review. Environ. Int. 124, 265–277 (2019)
J. Wu, B. Wang, L. Blaney et al., Degradation of sulfamethazine by persulfate activated with organo-montmorillonite supported nano-zero valent iron. Chem. Eng. J. 361, 99–108 (2019)
E. Justo-Cabrera, E. Flores-Rojas, D. Schnabel, Using nano zero valent iron supported on diatomite to remove acid blue dye: synthesis, characterization and toxicology test. bioRxiv (2019). https://doi.org/10.1101/755975v1.abstract
N. Arancibia-Miranda, S.E. Baltazar, A. García et al., Nanoscale zero valent supported by Zeolite and Montmorillonite: Template effect of the removal of lead ion from an aqueous solution. J. Hazard. Mater. 301, 371–380 (2016)
Y. Zou, X. Wang, A. Khan et al., Environmental remediation and application of nanoscale zero-valent iron and its composites for the removal of heavy metal ions: a review. Environ. Sci. Technol. 50, 7290–7304 (2016)
H. Dong, J. Deng, Y. Xie et al., Stabilization of nanoscale zero-valent iron (nZVI) with modified biochar for Cr(VI) removal from aqueous solution. J. Hazard. Mater. 332, 79–86 (2017)
B. Yang, Z. Tian, L. Zhang et al., Enhanced heterogeneous Fenton degradation of Methylene Blue by nanoscale zero valent iron (nZVI) assembled on magnetic Fe3O4/reduced graphene oxide. J. Water Process Eng. 5, 101–111 (2015)
G. Sheng, A. Alsaedi, W. Shammakh et al., Enhanced sequestration of selenite in water by nanoscale zero valent iron immobilization on carbon nanotubes by a combined batch, XPS and XAFS investigation. Carbon 99, 123–130 (2016)
A.N. Ay, B. Zumreoglu-Karan, A.G. Kalinichev et al., Layered double hydroxide–borate composites supported on magnetic nanoparticles: preparation, characterization and molecular dynamics simulations. J. Porous Mater. (2020). https://doi.org/10.1007/s10934-019-00853-4.pdf
K. Tanaka, N. Kozai, T. Ohnuki et al., Study on coordination structure of Re adsorbed on Mg–Al layered double hydroxide using X-ray absorption fine structure. J. Porous Mater. 26, 505–511 (2019)
O. Saber, H. Tagaya, Preparation of a new nano-layered materials and organic–inorganic nano-hybrid materials, Zn–Si LDH. J. Porous Mater. 16, 81–89 (2009)
N.J. Kang, D.Y. Wang, B. Kutlu et al., A new approach to reducing the flammability of layered double hydroxide (LDH)-based polymer composites: preparation and characterization of dye structure-intercalated LDH and its effect on the flammability of polypropylene-grafted maleic anhydride/d-LDH composites. ACS Appl. Mater. Interfaces 5(18), 8991–8997 (2013)
D. Voiry, J. Yang, J. Kupferberg et al., High-quality graphene via microwave reduction of solution-exfoliated graphene oxide. Science 353, 1413–1416 (2016)
E. Erdim, A.R. Badireddy, M.R. Wiesner, Characterizing reactive oxygen generation and bacterial inactivation by a zerovalent iron-fullerene nano-composite device at neutral pH under UV-A illumination. J. Hazard. Mater. 283, 80–88 (2015)
Y.P. Wei, D.Q. Wei, H.W. Gao, Treatment of dye wastewater by in situ hybridization with Mg–Al layered double hydroxides and reuse of dye sludge. Chem. Eng. J. 172, 872–878 (2011)
P. Gholami, L. Dinpazhoh, A. Khataee, A. Hassani, A. Bhatnagar, Facile hydrothermal synthesis of novel Fe-Cu layered double hydroxide/biochar nanocomposite with enhanced sonocatalytic activity for degradation of cefazolin sodium. J. Hazard. Mater. 381, 120742 (2019)
A. Khataee, T.S. Rad, S. Nikzat et al., Fabrication of NiFe layered double hydroxide/reduced graphene oxide (NiFe-LDH/rGO) nanocomposite with enhanced sonophotocatalytic activity for the degradation of moxifloxacin. Chem. Eng. J. 375, 122102 (2011)
H. Lyu, K. Hu, J. Fan et al., 3D hierarchical layered double hydroxide/carbon spheres composite with hollow structure for high adsorption of dye. Appl. Surf. Sci. 500, 144037 (2020)
Z.B. Jubri, N.Z.A.B.M. Yusoff, S.H.B. Sarijo et al., Synthesis, characterization and controlled release properties of zinc-aluminium-beta-naphthoxyacetate nanocomposite. J. Porous Mater. 24(3), 573–582 (2017)
M. Silion, D. Hritcu, G. Lisa et al., New hybrid materials based on layered double hydroxides and antioxidant compounds. Preparation, characterization and release kinetic studies. J. Porous Mater. 19, 267–276 (2012)
M. Liu, X. Zhu, Y. Wei et al., A novel hollowed-out Si microsphere encapsulated by graphene oxide: a strong and reusable absorbent. J. Porous Mater. (2020). https://doi.org/10.1007/s10934-020-00877-1.pdf
H. Song, S. Kim, Y. Cho, Removal of heavy metals using sorbents derived from bark. J. Porous Mater. 27, 319–328 (2020)
J. Li, M. Fan, M. Li et al., Cr(VI) removal from groundwater using double surfactant-modified nanoscale zero-valent iron (nZVI): effects of materials in different status. Sci. Total Environ. 717, 137112 (2020)
F. Zhu, S. He, T. Liu et al., Effect of pH, temperature and co-existing anions on the Removal of Cr(VI) in groundwater by green synthesized nZVI/Ni. Ecotoxicol. Environ. Saf. 163, 544–550 (2018)
Y. Fang, J. Wen, H. Zhang et al., Enhancing Cr(VI) reduction and immobilization by magnetic core-shell structured NZVI@MOF derivative hybrids. Environ. Pollut. 260, 114021 (2020)
M. Li, Y. Mu, H. Shang et al., Phosphate modification enables high efficiency and electron selectivity of nZVI toward Cr(VI) removal. Appl. Catal. B: Environ. 263, 118364 (2020)
H. Dong, J. Deng, Y. Xie, C. Zhang, G. Zeng, Stabilization of nanoscale zero-valent iron (nZVI) with modified biochar for Cr(VI) removal from aqueous solution. J. Hazard. Mater. 33215, 79–86 (2017)
S. Huang, L. Gu, N. Zhu et al., Heavy metal recovery from electroplating wastewater by synthesis of mixed-Fe3O4@SiO2/metal oxide magnetite photocatalysts. Green Chem. 16, 2696–2705 (2014)
F. Liu, Y. Lu, H. Chen et al., Removal of Cr6+ from groundwater using zero-valence iron in the laboratory. Chem. Speciat. Bioavailab. 14, 75–77 (2002)
H. Park, R.K. Sushil, C. Heechul, Arsenic removal by nano-scale zero valent iron and how it is affected by natural organic matter. Environ. Appl. Nanoscale Microscale React. Met. Part. (2009). https://doi.org/10.1021/bk-2009-1027.ch008
Acknowledgements
The study was supported by SDUT & Zibo City Integration Development Project (NO. 2019ZBXC152), the National Natural Science Foundation of China (No. 41877122), Shandong Province Major Science and Technology Innovation Projects (No. 2018CXGC1011) and National Science and Technology Major Project (2016ZX05062003).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhao, S., Fan, X., Yang, J. et al. Enhanced removal of Cr(VI) from wastewater by nanoscale zero valent iron supported on layered double hydroxides. J Porous Mater 27, 1701–1710 (2020). https://doi.org/10.1007/s10934-020-00947-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-020-00947-4