Abstract
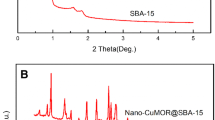
In this paper, the synthesis of core shell structured NiO@MCM-41 nanocomposite via vesicles as soft template is reported for the first time. Its catalytic performance was investigated in the CO2 reforming of methane (CRM) conversion. Stable vesicles first formed with CTAB/SDBS surfactant ratio of 1:2. Nickle nitrate was added to the vesicle mixture followed by addition of the aqueous solution of vesicle containing Ni cations inside to the MCM-41 gel. After high-temperature calcination, NiO@MCM-41 nanocomposite were obtained. The structural symmetry and the surface morphology were characterized by transmission electron microscope (TEM), low angle X-Ray Diffraction (XRD) and N2 adsorption/desorption analysis. TEM image confirmed core–shell structure and the hexagonally ordered structure of shell of MCM-41 silica. The results indicated that the average diameter of synthesized core–shell NiO@MCM-41 particles is 70–80 nm and the most of them are of spherical shape. The result of small angle XRD and N2 isotherm adsorption/desorption analyses indicated successfull formation of mesoporous shell. Hydrogen consumption by the catalyst mainly at 700 °C in TPR profile showed the strong interaction of the most of Nickel content with the support. CRM conversion on the prepared catalyst after 245 min of reaction led to H2 conversion at 42%, CO2 conversion at 48% with H2/CO yield ratio of 0.8.








Similar content being viewed by others
References
B.L. Cushing, V.L. Kolesnichenko, C.J. O’Connor, Recent advances in the liquid-phase syntheses of inorganic nanoparticles. Chem. Rev. 104, 3893–3946 (2004)
R. Davies et al. Engineered particle surfaces. Adv. Mater. 10(15), 1264–1270 (1998)
C. Hofman-Caris, Polymers at the surface of oxide nanoparticles. New J. Chem. 18(10), 1087–1096 (1994)
L.M. Liz-Marzán, M. Giersig, P. Mulvaney, Synthesis of nanosized gold-silica core–shell particles. Langmuir 12(18), 4329–4335 (1996)
R. Partch, In Material Synthesis and Characterization, D. Perry (ed.) (Plenum, New York, 1997)
D. Wilcox et al., Hollow and Solid Spheres and Microspheres. (Materials Research Society, Pittsburg, 1995)
D.V. Goia, E. Matijević, Preparation of monodispersed metal particles. New J. Chem. 22(11), 1203–1215 (1998)
M. Ohmori, E. Matijević, Preparation and properties of uniform coated inorganic colloidal particles: 8. Silica on iron. J. Colloid Interface Sci. 160(2), 288–292 (1993)
R. Partch, S. Brown, Aerosol and solution modification of particle-polymer interfaces. J. Adhes. 67(1–4), 259–276 (1998)
F. Wang, L. Xu, W. Shi, Syngas production from CO2 reforming with methane over core-shell Ni@ SiO2 catalysts. J CO2 Utilization 16, 318–327 (2016)
S.-J. Oh et al., Newly designed Cu/Cu10Sn3 core/shell nanoparticles for liquid phase-photonic sintered copper electrodes: large-area, low-cost transparent flexible electronics. Chem. Mater. 28(13), 4714–4723 (2016)
Z. Zhou et al., Preparation of calcium carbonate@graphene oxide core–shell microspheres in ethylene glycol for drug delivery. Ceram. Int. 42(2), 2281–2288 (2016)
L.E. Sperling et al., Advantages and challenges offered by biofunctional core–shell fiber systems for tissue engineering and drug delivery. Drug Discov Today 21(8), 1243–1256 (2016)
Z. Shen et al., Self-assembly of core-polyethylene glycol-lipid shell (CPLS) nanoparticles and their potential as drug delivery vehicles. Nanoscale 8(31), 14821–14835 (2016)
D. Saikia et al., Aqueous synthesis of highly stable CdTe/ZnS Core/Shell quantum dots for bioimaging. Luminescence 32(3), 401–408 (2016)
L. Tan et al., Chitosan-based core-shell nanomaterials for pH-triggered release of anticancer drug and near-infrared bioimaging. Carbohydr. Polym. 157, 325–334 (2017)
R.A. Perez, H.-W. Kim, Core–shell designed scaffolds for drug delivery and tissue engineering. Acta Biomater 21, 2–19 (2015)
E. Yan et al., Degradable polyvinyl alcohol/poly (butylene carbonate) core–shell nanofibers for chemotherapy and tissue engineering. Mater. Lett. 167, 13–17 (2016)
H.S.A. El-Haleem et al., Manganese dioxide-core–shell hyperbranched chitosan (MnO2–HBCs) nano-structured screen printed electrode for enzymatic glucose biosensors. RSC Adv. 6(110), 109185–109191 (2016)
C. Zhang et al., Electrochemical biosensor based on nanoporous Au/CoO core–shell material with synergistic catalysis. ChemPhysChem. 17(1), 98–104 (2016)
R. Zeng et al., Aqueous synthesis of type-II CdTe/CdSe core–shell quantum dots for fluorescent probe labeling tumor cells. Nanotechnology 20(9), 095102 (2009)
A. Haghtalab, A. Mosayebi, Co@ Ru nanoparticle with core–shell structure supported over γ-Al2O3 for Fischer–Tropsch synthesis. Int. J. Hydrogen Energy 39(33), 18882–18893 (2014)
A. Shafiefarhood et al., Fe2O3@ LaxSr1 –xFeO3 core–shell redox catalyst for methane partial oxidation. ChemCatChem 6(3), 790–799 (2014)
J.-X. Feng et al., Caffeine-assisted facile synthesis of platinum@ palladium core-shell nanoparticles supported on reduced graphene oxide with enhanced electrocatalytic activity for methanol oxidation. Electrochim. Acta 142, 343–350 (2014)
M. Zhang et al., Improved catalytic activity of cobalt core–platinum shell nanoparticles supported on surface functionalized graphene for methanol electro-oxidation. Electrochim. Acta 158, 81–88 (2015)
M.A. Lucchini et al., Sintering and coking resistant core–shell microporous silica–nickel nanoparticles for CO methanation: towards advanced catalysts production. Appl. Catal. B 182, 94–101 (2016)
W. Qi et al., Core–shell nanostructures: modeling, fabrication, properties, and applications. J. Nanomaterials 2012, 2 (2012)
J. Hao et al., Zn2+-induced vesicle formation. J. Phys. Chem. B 108(4), 1168–1172 (2004)
W. Song et al., Synthesis of magnetic core–shell structure Fe3O4@ MCM-41 nanoparticle by vesicles in aqueous solutions. Chin. J. Chem. Eng. 23(8), 1398–1402 (2015)
N. Zhao et al., Controlled synthesis of gold nanobelts and nanocombs in aqueous mixed surfactant solutions. Langmuir 24(3), 991–998 (2008)
L. Fan, R. Guo, Growth of dendritic silver crystals in CTAB/SDBS mixed-surfactant solutions. Cryst. Growth Des. 8(7), 2150–2156 (2008)
F. Liu et al., Transcriptive synthesis of Mg(OH)2 hollow nanospheres and the non-equilibrium shell fusion assisted by catanionic vesicles. J. Phys. Chemis. B 113(33), 11362–11366 (2009)
D.H. Kim et al., The catalytic stability of TiO2-shell/Ni-core catalysts for CO2 reforming of CH4. Appl. Catal. A 495, 184–191 (2015)
X. Zheng et al., LaNiO3@ SiO2 core–shell nano-particles for the dry reforming of CH4 in the dielectric barrier discharge plasma. Int. J. Hydrogen Energy 39(22), 11360–11367 (2014)
J. Zhang, F. Li, Coke-resistant Ni@ SiO2 catalyst for dry reforming of methane. Appl. Catal. B 176, 513–521 (2015)
E. Baktash et al., Alumina coated nickel nanoparticles as a highly active catalyst for dry reforming of methane. Appl. Catal. B 179, 122–127 (2015)
P. Ferreira-Aparicio, A. Guerrero-Ruiz, I. Rodrıguez-Ramos, Comparative study at low and medium reaction temperatures of syngas production by methane reforming with carbon dioxide over silica and alumina supported catalysts. Appl. Catal. A 170(1), 177–187 (1998)
J. Edwards, A. Maitra, The chemistry of methane reforming with carbon dioxide and its current and potential applications. Fuel Process. Technol. 42(2–3), 269–289 (1995)
M. Bradford, M. Vannice, CO2 reforming of CH4. Catal. Rev. 41(1), 1–42 (1999)
J. Rostrupnielsen, J.B. Hansen, CO2-reforming of methane over transition metals. J. Catal. 144(1), 38–49 (1993)
X. Gao et al., Highly reactive Ni-Co/SiO2 bimetallic catalyst via complexation with oleylamine/oleic acid organic pair for dry reforming of methane. Catal. Today 281, 250–258 (2017)
J.R. Rostrup-Nielsen, Industrial relevance of coking. Catal. Today 37(3), 225–232 (1997)
H. Swaan et al., Deactivation of supported nickel catalysts during the reforming of methane by carbon dioxide. Catal. Today 21(2), 571–578 (1994)
L. Neal, A. Shafiefarhood, F. Li, Effect of core and shell compositions on MeOx@ LaySr1–yFeO3 core–shell redox catalysts for chemical looping reforming of methane. Appl. Energy 157, 391–398 (2015)
J. Liu, R.E. Saw, Y.-H. Kiang, Calculation of effective penetration depth in X-ray diffraction for pharmaceutical solids. J. Pharm. Sci. 99(9), 3807–3814 (2010)
D.W. Fitting, W.P. Dube, T.A. Siewert, High energy x-ray diffraction technique for monitoring solidification of single crystal castings. Nondestruct. Charact. Mater. 8, 217–222 (1998)
M. OOKAWA et al., X-ray diffraction analysis of ordered mesoporous silica. Stud. Surf. Sci. Catal. 135(1), 198–198 (2001)
Q. Zhang et al., A sintering and carbon-resistant Ni-SBA-15 catalyst prepared by solid-state grinding method for dry reforming of methane. J. CO2 Utilization 17, 10–19 (2017)
A.J. Majewski, J. Wood, W. Bujalski, Nickel–silica core@shell catalyst for methane reforming. Int. J. Hydrogen Energy 38(34), 14531–14541 (2013)
A. Loaiza-Gil et al., Thermal decomposition study of silica-supported nickel catalyst synthesized by the ammonia method. J. Mol. Catal. A: Chem. 281(1), 207–213 (2008)
Y. Matsumura, T. Nakamori, Steam reforming of methane over nickel catalysts at low reaction temperature. Appl. Catal., A 258(1), 107–114 (2004)
Y.-X. Pan, C.-J. Liu, P. Shi, Preparation and characterization of coke resistant Ni/SiO2 catalyst for carbon dioxide reforming of methane. J. Power Sour. 176(1), 46–53 (2008)
H.-W. Chen et al., Carbon dioxide reforming of methane reaction catalyzed by stable nickel copper catalysts. Catal. Today 97(2), 173–180 (2004)
A. Djaidja et al., Characterization and activity in dry reforming of methane on NiMg/Al and Ni/MgO catalysts. Catal. Today 113(3), 194–200 (2006)
L.-j. SI et al., Influence of preparation conditions on the performance of Ni–CaO–ZrO2 catalysts in the tri-reforming of methane. J. Fuel Chem. Technol. 40(2), 210–215 (2012)
P. Jin et al., Synthesis and catalytic properties of nickel–silica composite hollow nanospheres. J. Phys. Chem. B 108(20), 6311–6314 (2004)
P. Burattin, M. Che, C. Louis, Characterization of the Ni(II) phase formed on silica upon deposition–precipitation. J. Phys. Chem. B 101(36), 7060–7074 (1997)
T. Lehmann et al., Physico-chemical characterization of Ni/MCM-41 synthesized by a template ion exchange approach. Microporous Mesoporous Mater. 151, 113–125 (2012)
J.C. Park et al., Chemical transformation and morphology change of nickel–silica hybrid nanostructures via nickel phyllosilicates. Chem. Commun. 2009(47), 7345–7347
M. Ocana, A. Gonzalez-Elipe, Preparation and characterization of uniform spherical silica particles coated with Ni and Co compounds. Colloids Surf. A 157(1), 315–324 (1999)
P. Burattin, M. Che, C. Louis, Molecular approach to the mechanism of deposition–precipitation of the Ni(II) phase on silica. J. Phys. Chem. B 102(15), 2722–2732 (1998)
P. Burattin, M. Che, C. Louis, Metal particle size in Ni/SiO2 materials prepared by deposition–precipitation: Influence of the nature of the Ni(II) phase and of its interaction with the support. J. Phys. Chem. B 103(30), 6171–6178 (1999)
P. Burattin, M. Che, C. Louis, Ni/SiO2 materials prepared by deposition–precipitation: influence of the reduction conditions and mechanism of formation of metal particles. J. Phys. Chem. B 104(45), 10482–10489 (2000)
Acknowledgements
Authors gratefully acknowledge the financial support of Iran Nanotechnology Initiative Council (INIC).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roosta, Z., Izadbakhsh, A., Sanati, A.M. et al. Synthesis and evaluation of NiO@MCM-41 core–shell nanocomposite in the CO2 reforming of methane. J Porous Mater 25, 1135–1145 (2018). https://doi.org/10.1007/s10934-017-0525-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-017-0525-8