Abstract
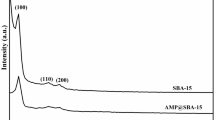
A polycrown loaded silica composite material named Polycrown/SiO2-P was firstly prepared by impregnating and immobilizing polycrown into porous silica and was characterized by scanning electron microscope (SEM), and Fourier transformed infrared (FT-IR) spectroscopy, nitrogen adsorption and desorption isotherms. Polycrown/SiO2-P was tested with regard to adsorption performance. Interestingly, Polycrown/SiO2-P preferred Pd(II) than alkali metals, alkaline-earth metals and actinides. Pd(II) adsorption with Polycrown/SiO2-P as functions of HNO3 concentration, contact time, initial Pd(II) concentration, temperature were thus investigated. The results showed the optimal adsorption acidity was determined as 5.0 M nitric acid, proving good acidity-resistant. Pseudo-second-order kinetics model fitted adsorption well indicating chemical adsorption. The isotherm adsorption equilibrium was well described by Langmuir isotherm model with maximum adsorption capacity of 14.30 mg/g. Pd(II) adsorption increased with temperature increased. The positive ΔH° and ΔS°, and the negative ΔG° indicated the adsorption was endothermic, entropy increase and spontaneous in nature. Pd(II) loaded Polycrown/SiO2-P was characterized by XPS. Further, the adsorption efficiency of recovered Polycrown/SiO2-P retained up ~93.7% after five ad-desorption cycles. Polycrown/SiO2-P was promising to separate Pd(II) from highly acid medium.








Similar content being viewed by others
References
M. Seltzer, R. Michel, Preparation of electrodeless discharge lamps for atomic fluorescence spectrometry. Anal. Chem. 55, 1817–1819 (1983)
H. Tan, I. Houpis, R. Liu, Y. Wang, Z. Chen, M.J. Fleming, Palladium-catalyzed coupling of sulfonylhydrazones with heteroaromatic 2-amino-halides (barluenga reaction): exploring the electronics of the sulfonylhydrazone. Org. Process. Res. Dev. 19, 1044–1048 (2015)
E. Yavuz, Ş. Tokalıoğlu, H. Şahan, Ş. Patat, Nano sponge Mn2O3 as a new adsorbent for the preconcentration of Pd(II) andRh(III) ions in sea water, wastewater, rock, street sediment and catalytic converter samples prior to FAAS determinations. Talanta 128, 31–37 (2014)
S. Barson, P. Skeldon, G. Thompson, A. Kolitsch, E. Richter, X. Wieser, J. Piekoszewski, A. Chmielewski, Z. Werner, Investigation of ion assisted palladium treatments for improved corrosion resistance of titanium foil in the electron beam dry scrubber process. Surf. Coat. Technol. 127, 179–192 (2000)
J. Tsuji, Palladium reagents and catalysts: new perspectives for the 21st century, Wiley (2004)
B. Lim, M. Jiang, J. Tao, P. Camargo, Y. Zhu, Y. Xia, Shape-controlled synthesis of Pd nanocrystals in aqueous solutions. Adv. Funct. Mater. 19, 189–200 (2009)
A. Milheiro, J. Muris, C.J. Kleverlaan, A.J. Feilzer, Influence of shape and finishing on the corrosion of palladium-based dental alloys. J. Adv. Prosthodont 7, 56–61 (2015)
M. Ruiz, A.M. Sastre, E. Guibal, Palladium sorption on glutaraldehyde-cross-linked chitosan. React. Funct. Polym. 45, 155–173 (2000)
Z. Kolarik, E.V. Renard, Potential applications of fission platinoids in industry. Platin. Met. Rev. 49, 79–90 (2005)
M. Aghaie, M. Giahi, H. Aghaie, M. Arvand, M. Pournaghdy, F. Yavari, New Fe(II) ion-selective electrode based on N-phenylaza-15-crown-5 as neutral carrier in PVC matrix. Desalination 247, 346–354 (2009)
R. Addleman, J. Bennett, S. Tweedy, S. Elshani, C. Wai, Response of a benzoxainone derivative linked to monoaza-15-crown-5 with divalent heavy metals. Talanta 46, 573–581 (1998)
G. Rounaghi, Selective uranyl cation detection by polymeric ion selective electrode based on benzo-15-crown-5. Mater. Sci. Eng. C 31, 1637–1642 (2011)
H. Hama, T. Morozumi, H. Nakamura, Novel Mg2+-responsive fluorescent chemosensor based on benzo-15-crown-5 possessing 1-naphthaleneacetamide moiety. Tetrahedron Lett. 48, 1859–1861 (2007)
A.E. Osmanlioqlu, Removal of radioactive contaminants by polymeric microspheres. Environ. Technol. 37, 2830–2834 (2016)
B.S. Mohite, A.S. Jadhav, Column chromatographic separation of uranium(VI) and other elements using poly(dibenzo-18-crown-6) and ascorbic acid medium. J. Chromatogr. A 983, 277–281 (2003)
S.N. Lad, B.S. Mohite, Analytical application of poly[dibenzo-18-crown-6] for column chromatographic separation of Nd(III) from Ce(III), U(VI) and other elements. J. Radioanal. Nucl. Ch. 302, 1481–1487 (2014)
B.S. Mohite, D.N. Zambare, B.E. Mahadik, Separation of barium from associated elements using poly(dibenzo-18-crown-6) and column chromatography. Analyst 119, 2033–2036 (1994)
W. Zhang, A. Zhang, L. Xu, Z. Chai, Extraction of cesium and some typical metals with a supramolecular recognition agent 1,3-di(1-nonyloxy) -2,4-crown-6- calix [4] arene. J. Chem. Eng. Data 58, 167–175 (2013)
A. Zhang, J. Jiang, Z. Chai, Preparation of a macroporous silica - based multidentate soft-ligand material and its application in the adsorption of palladium and the others. Sep. Sci. Technol. 48, 1500–1509 (2013)
A. Zhang, Y. Zhu, Z. Chai, New insight into the partitioning of minor actinides i: extraction of palladium and some typical metals with a multidentate soft-ligand 2,6- bis(5,6- dinonyl- 1,2,4- triazine- 3- yl)pyridine. J. Chem. Eng. Data 57, 1267–1273 (2012)
A. Zhang, Q. Hu, Adsorption of cesium and some typical coexistent elements onto a modified macroporous silica-based supramolecular recognition material. Chem. Eng. J. 159, 58–66 (2010)
A. Zhang, W. Wang, Z. Chai, M. Kumagai, Separation of strontium ions from a simulated highly active liquid waste using a composite of silica-crown ether in a polymer. J. Sep. Sci. 31, 3148–3155 (2008)
C. Xiao, A. Zhang, Z. Chai, Synthesis and characterization of novel macroporous silica-polymer-calixcrown hybrid supramolecular recognition materials for effective separation of cesium. J. Hazard. Mater 267, 109–118 (2014)
K. Otake, T. Suzuki, H. Kim, M. Nomura, Y. Fujii, Novel syntheses method of phenol type benzo-15-crown-5 ether resin and its application for lithium isotope separation. J. Nucl. Sci. Technol. 4, 419–422(2006)
Z. Zhang, J. Liu, X. Cao, X. Luo, R. Hua, Y. Liu, X. Yu, L. He, Y. Liu, Comparison of U(VI) adsorption onto nanoscale zero-valent iron and red soil in the presence of U(VI)-CO3/Ca-U(VI)-CO3 complexes. J. Hazard. Mater 300, 633–642 (2015)
A. Zhang, Y. Wei, M. Kumagai, Synthesis of a novel macroporous silica-based polymeric material containing 4, 4′,(5′)-di(tert-butylcyclohexano)-18-crown-6 functional group and its adsorption mechanism for strontium. React. Funct. Polym 61, 191–202 (2004)
Y. Wei, M. Kumagai, Y. Takashima, R. Odoj, Studies on the separation of minor actinide from high-level wastes by extraction hromatography using novel silica-based extraction resins. Nucl. Technol. 132, 413–423 (2000)
A. Zhang, Z. Chai, Adsorption property of cesium onto modified macroporous silica-calix [4] arene-crown based supramolecular recognition materials. Ind. Eng. Chem. Res 51, 6196–6204 (2012)
M.G. Sujana, S. Anand, Iron and aluminium based mixed hydroxides: a novel sorbent for fluoride removal from aqueous solutions. Appl. Surf. Sci 256, 6956–6962 (2010)
K. Hong, S. Sun, W. Tian, G.Q. Chen, W. Huang, A rapid method for detecting bacterial polyhydroxyalkanoates in intact cells by Fourier transform infrared spectroscopy. Appl. Microbiol. Biot 51, 523–526 (1999)
J.R. Dai, Y.F. Hallock, J.H. Cardellina, M.R. Boyd, Hiv-inhibitory and cytotoxic oligostilbenes from the leaves of hopea malibato. J. Nat. Prod 61, 351–353 (1998)
V. Ermini, E. Finocchio, S. Sechi, G. Busca, S. Rossini, An FT-IR and flow reactor study of the conversion of propane on γ-Al2O3 in oxygen-containing atmosphere. Appl. Catal. A Gen. 190, 157–167 (2000)
N. Rouhi, B. Esfandyarpour, S. Mohajerzadeh, P. Hashemi, M.D. Robertson, K. Raffel, Low temperature growth of silicon dioxide using hydrogenation assisted nano-crystallization and plasma enhanced oxidation. J. Non-Cryst. Solids 356, 1027–1031 (2010)
R.K. Nariyal, P. Kothari, B. Bisht, FTIR measurements of SiO2 glass prepared by sol–gel technique. Chem. Sci. Trans. 3, 1064–1066 (2014)
A. Zhang, C. Xiao, E. Kuraoka, M. Kumagai, Molecular modification of a novel macroporous silica-based impregnated polymeric composite by tri-n-butyl phosphate and its application in the adsorption for some metals contained in a typical simulated HLLW. J. Hazard. Mater 147, 601–609 (2007)
C. Xiao, A. Zhang, Z. Chai, Synthesis and characterization of novel macroporous silica- polymer-calixcrown hybrid supramolecular recognition materials for effective separation of cesium. J. Hazard. Mater 267, 109–118 (2014)
J. Han, N. Song, X. Tian, Synthesis and structure analysis of sandwich complexes of potassium and cesium with benzo-15-crown-5 derivatives. J. Incl. Phenom. Macr. 77, 301–308 (2013)
S. Alexandratos, C. Stine, Synthesis of ion-selective polymer supported crown ethers: a review. React. Funct. Polym 60, 3–16 (2004)
M. Yurdakoç, Y. Seki, S. Karahan, K. Yurdakoç, Kinetic and thermodynamic studies of boron removal by Siral 5, Siral 40, and Siral 80. J. Colloid. Interf. Sci 286, 440–446 (2005)
L. Zhou, J. Liu, Z. Liu, Adsorption of platinum(IV) and palladium(II) from aqueous solution by thiourea-modified chitosan microspheres. J. Hazard. Mater 172, 439–446 (2009)
J. Simonin, On the comparison of pseudo-first order and pseudo-second order rate laws in the modeling of adsorption kinetics. Chem. Eng. J 300, 254–263 (2016)
R. Hu, X. Wang, S. Dai, D. Shao, T. Hayat, A. Alsaedi, Application of graphitic carbon nitride for the removal of Pb(II) and aniline from aqueous solutions. Chem. Eng. J 260, 469–477 (2015)
V. Gallardo, R. Navarro, I. Saucedo, M. Avila, E. Guibal, Zinc(II) extraction from hydrochloric acid solutions using Amberlite XAD-7 impregnated with Chyphos IL101 tetradecyl (trihexyl) phosphonium chloride). Sep. Sci. Technol. 43, 2434–2459 (2008)
A. Nilchi, R. Saberi, M. Moradi, H. Azizpour, R. Zarghami, Adsorption of caesium on copper hexacyanoferrate-PAN composite ion exchanger from aqueous solution. Chem. Eng. J 172, 572–580 (2001)
J. Moon, C. Jung, B. Lee, C. Shin, Adsorptive separation of palladium from a simulated nuclear waste solution with activated carbon fibers. Sep. Sci. Technol. 43, 567–581 (2008)
C. Wanger, W. Riggs, L. Davis, J. Moulder, G. Muilenberg, Handbook of X-ray photoelectron spectroscopy, Published by Perkin-Elmer Corp., USA, (1979)
Acknowledgments
The present work was financially supported by National Natural Science Foundation of China (No. 11605027, No. U1407115 and No. 91126021), the Project of the Jiangxi Provincial Department of Education (Grant No. GJJ150560), the Doctoral Research Fund of East China University of Technology (Grant No.DHBK2015301), the Natural Science Foundation of Jiangxi Province (No.20151BDH80073), the Research Foundation of Education Bureau of Jiangxi Province (No. GJJ150566) and the Zhejiang Provincial Natural Science Foundation of China (No.LY17B060004).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dai, Y., Liu, Y. & Zhang, A. Preparation and characterization of a mesoporous polycrown impregnated silica and its adsorption for palladium from highly acid medium. J Porous Mater 24, 1037–1045 (2017). https://doi.org/10.1007/s10934-016-0343-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-016-0343-4