Abstract
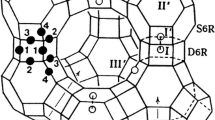
The single-crystals of Ca2+, K+-exchanged zeolite Y, and Ca2+, Rb+-exchanged zeolite Y were prepared by using flow method with mixed ion-exchange solution, whose Ca(NO3)2:KNO3 mole ratios were 1:1 (crystal 1) and 1:100 (crystal 2), and Ca(NO3)2:RbNO3 mole ratios were 1:1 (crystal 3) and 1:100 (crystal 4), respectively, with a total concentration of 0.05 M. They were fully dehydrated by vacuum dehydration at 723 K and 1 × 10−6 Torr for 2 days. Their crystals were determined by single-crystal synchrotron X-ray diffraction techniques in the cubic space group \(Fd \overline{3}\) m, respectively, and were refined to the final error indices R 1/wR 2 = 0.057/0.196, 0.073/0.223, 0.055/0.188, and 0.049/0.175 for crystals 1, 2, 3, and 4, respectively. In the structure of crystal 1 (|Ca23K29|[Si117Al75O384]-FAU), 23 Ca2+ ions per unit cell occupy sites I, II′, and II; 29 K+ ions per unit cell are at sites II′, II, and III′. In the structure of crystal 2 (|Ca18.5K38|[Si117Al75O384]-FAU), 18.5 Ca2+ ions per unit cell occupy sites I, I′, and II; 38 K+ ions are at sites I′, II, and III′. In the structure of crystal 3 (|Ca27Rb21|[Si117Al75O384]-FAU), 27 Ca2+ ions per unit cell occupy sites I, II′, and II; 21 Rb+ ions per unit cell are at sites II′, II, and III. In the structure of crystal 4 (|Ca18Rb39|[Si117Al75O384]-FAU), 18 Ca2+ ions per unit cell occupy sites I and II; 39 Rb+ ions per unit cell are at sites I′, II′, II, III, and III′. In the four crystals, the Ca2+ ion which has much smaller size and higher charge than other cations such as K+ and Rb+ energetically preferred at site I and so the first to be filled on it. Unlike Ca2+ ion, no K+ and Rb+ ions are found at site I, which are clearly less favorable for K+ and Rb+ ions.




Similar content being viewed by others
References
S. Sano, T. Maruo, H. Yamatera, M. Suzuki, Y. Saito, J. Am. Chem. Soc. 109, 52 (1987)
T.I. Koranyi, N.H. Pham, A. Jentys, H. Vinek, Stud. Surf. Sci. Catal. 106, 509 (1997)
I. Smiciklas, S. Dimovic, I. Plecas, Appl. Clay Sci. 35, 139 (2007)
S.B. Jang, Y. Kim, Bull. Korean Chem. Soc. 16, 539 (1995)
S.M. Seo, W.T. Lim, K. Seff, Micro. Meso. Mater. 170, 67 (2013)
H.S. Kim, D. Bae, W.T. Lim, K. Seff, J. Phys. Chem. C 116, 9009 (2012)
Y.F. Shepelev, I.K. Butikova, Y.I. Smolin, Zeolites 11, 287 (1991)
S.H. Lee, Y. Kim, D.S. Kim, K. Seff, Bull. Korean Chem. Soc. 19, 98 (1998)
K.S. Ryu, M.N. Bae, Y. Kim, K. Seff, Micro. Meso. Mater. 71, 65 (2004)
M.N. Bae, Bull. Korean Chem. Soc. 28, 251 (2007)
Y.H. Yeom, S.B. Jang, Y. Kim, S.H. Song, K. Seff, J. Phys. Chem. B 101, 6914 (1997)
A.A. Anderson, Y.F. Shepelev, Y.I. Smolin, Zeolites 10, 32 (1990)
Y.I. Smolin, Y.F. Shepelev, A.A. Anderson, Acta Crystallogr. Sect. B 45, 124 (1989)
D.H. Olson, E. Dempsey, J. Catal. 13, 221 (1969)
S.B. Jang, M.S. Jeong, Y. Kim, K. Seff, J. Phys. Chem. B 101, 9041 (1997)
S.B. Jang, M.S. Jeong, Y.W. Han, Y. Kim, Bull. Korean Chem. Soc. 17, 631 (1996)
M.N. Bae, M.K. Song, Y. Kim, Bull. Korean Chem. Soc. 22, 1081 (2001)
G.H. Jeong, Y. Kim, Bull. Korean Chem. Soc. 23, 1121 (2002)
Y. Kim, K. Seff, Bull. Korean Chem. Soc. 5, 117 (1984)
Y. Kim, S.H. Song, D.S. Kim, Y.W. Han, D.K. Park, J. Korean Chem. Soc. 33, 18 (1989)
Y.S. Song, U.S. Kim, Y. Kim, D.S. Kim, Bull. Korean Chem. Soc. 11, 328 (1990)
N.H. Heo, Y. Kim, Bull. Korean Chem. Soc. 11, 407 (1990)
S.B. Jang, S.H. Song, Y. Kim, J. Korean Chem. Soc. 39, 7 (1995)
S.B. Jang, S.H. Song, Y. Kim, J. Korean Chem. Soc. 40, 427 (1996)
J.H. Kwon, S.B. Jang, Y. Kim, J. Phys. Chem. B 100, 13720 (1996)
D.W. Breck, Zeolite Molecular Sieves (Wiley, New York, 1973), pp. 92–107
W.T. Lim, S.Y. Choi, J.H. Choi, Y.H. Kim, N.H. Heo, K. Seff, Micro. Meso. Mater. 92, 234 (2006)
W.T. Lim, S.M. Seo, L. Wang, G.Q. Lu, K. Seff, Micro. Meso. Mater. 129, 11 (2010)
A.J. Arvai, C. Nielsen, ADSC quantum-210 ADX program (Area Detector System Corporation, Poway, 1993)
W. Minor, M. Cymborowski, Z. Otwinowski, M. Chruszcz, Acta Crystallogr. Sect. D 62, 859 (2006)
Bruker-AXS (ver. 6.12), XPREP, Program for the Automatic Space Group Determination. (Bruker AXS Inc., Madison, 2001)
G.M. Sheldrick, SHELXL97 Program for the Refinement of Crystal Structures (University of Gottingen, Gottingen, 1997)
P.A. Doyle, P.S. Turner, Acta Crystallogr. Sect. A 24, 390 (1968)
J.A. Ibers, W.C. Hamilton (eds.), International Tables for X-ray Crystallography, vol. IV (Kynoch Press, Birmingham, 1974), pp. 71–98
D.T. Cromer, Acta Crystallogr. 18, 17 (1965)
J.A. Ibers, W.C. Hamilton (eds.), International Tables for X-ray Crystallography, vol. IV (Kynoch Press, Birmingham, 1974), pp. 148–150
W. Loewenstein, Am. Mineral. 39, 92 (1954)
D.W. Breck, Zeolite Molecular Sieves (Wiley, New York, 1974), pp. 93–103
H. Van Bekkum, E.M. Flanigen, P.A. Jacobs, J.C. Jansen, Introductions to Zeolite Science and Practice (Elsevier, Amsterdam, 2001), p. 44
R.C. Weast, in Handbook of Chemistry and Physics, 70th edn. (The Chemical Rubber Co., Cleveland, 1989/1990), p. F-187
S.M. Seo, O.S. Lee, H.S. Kim, D.H. Bae, I. Chun, W.T. Lim, Bull. Korean Chem. Soc. 28, 1675 (2007)
Acknowledgements
The authors wish to thank the staff at Beamline 2D SMC at the Pohang Light Source, Korea, for assistance during data collection. This research was supported by GAIA project from Korea Environmental Industry and Technology Institute (KEITI 2015000550008).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kim, H.S., Kim, Y.H. & Lim, W.T. Crystallographic studies on the site selectivity of Ca2+, K+, and Rb+ ions within zeolite Y (Si/Al = 1.56). J Porous Mater 24, 959–972 (2017). https://doi.org/10.1007/s10934-016-0335-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-016-0335-4