Abstract
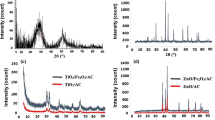
Activated carbon (AC) electrodes were loaded with TiO2 by using sol–gel method after a pretreatment process, the effect of the acidic pretreatment of the TiO2 loaded electrode on its deionization efficiency of the wastewater containing NaCl solutions was studied; the physical, chemical and electrochemical properties of the electrode were characterized. The physical and chemical properties of the activated carbon before and after loaded with TiO2 nanoparticles are characterized by using scanning electron microscopy, energy dispersion spectrum analyzer, Brunauer–Emmett–Teller gas adsorption method, thermal gravimetric analysis, Fourier transform infrared spectroscopy respectively . Electrochemical properties were characterized by employing electrochemical workstation and electrical adsorption deionization test. It was found that both the specific capacitance and the ions removal efficiency of the activated carbon loaded with TiO2 had an increase of 16.4 and 49.8 % respectively in comparison with original activated carbon electrode. It was believed that this is due to the presence of crystal anatase TiO2 nanoparticles (the mass content of titanium element in the TiO2/AC complex is about 24.91 %) on the surface and pores in the activated carbon; while Ti–O–C bonds was found on the surface of the activated carbon, its surface wetting properties was significantly improved. However, it was also noticed that and the specific surface area of the activated carbon was decreased from 680.5 to 523.35 m2 g−1. This might lead to the decrease of the physical adsorption properties of the activated carbon electrodes, but its Electrical double-layer capacitance increases, electrical adsorption efficiency was improved.









Similar content being viewed by others

References
C.M. Yang, W.H. Choi, B.K. Na et al., Capacitive deionization of NaCl solution with carbon aerogel-silica gel composite electrodes. Desalination 174(2), 125–133 (2005)
Y. Guangjun, C. Fuming, Progress in capacitive deionization. Technol. Water Treat. 2(29), 63–66 (2003)
T.J. Welgemoeda, C.F. Schutte, Capacitive deionization technologytm: an alternative desalination solution. Desalination 183, 327–340 (2005)
K.K. Park, J.B. Lee, P.Y. Park et al., Development of a carbon sheet electrode for electrosorption desalination. Desalination 206(1), 86–91 (2007)
M.W. Ryoo, G. Seo, Improvement in capacitive deionization function of activated carbon cloth by titania modification. Water Res. 37, 1527–1534 (2003)
J.C. Farmer, D.V. Fix, G.V. Mack, Capacitive deionization with carbon aerogel electrodes:carbonate, sulfate, and phosphate. Int. SAMPE Technical. Conf. 27(2), 294–304 (1995)
J.C. Farmer, D.V. Fix, G.V. Mack et al., Capacitive deionization of NaCl and NaNO3 solutionswith carbon aerogel electrodes. J. Electrochem. Soc. 143(1), 159–169 (1996)
X. Wang, M. Li, Electrosorption of ions from aqueous solutions with carbon nanotubes and nanofibers composite film electrodes. Appl. Phys. Lett. 89(5), 053127.1–053127.3 (2006)
L. Zhang, J. Hu, S. Zhao, J. Liu, ReCent advances of research on modifled AC tivatde carbon. Mater. Rev. 11(23), 435–438 (2009)
L.T. Li, Q. Xie et al., Preparation and characterization of metal-containing activated carbon for electrode. J. China Univ. Min. Technol. 2(37), 225–230 (2008)
A. Lisovskii, R. Semiat, C. Aharonl, Adsorption of sulfur dioxide by active carbon treated by nitric acide: effect of the treatment on adsorption of SO2 and extratability of the acid foemed. Carbon 35(10–11), 1638–1643 (1997)
A. Morwski, M. Inagki, Application of modified synthetic catbon for adsorption of trihalomethanes from water. Desalination 114, 23–27 (1997)
K. Laszlo, A. Szucs, Surface characterization of PET based activated carbon and the effect of pH on its adsorption capacity from aqueous phenol and 2,2,4-trichlorophenol solutions. Carbon 39, 1945–1953 (2001)
L.Y.W. Yili, Removal of copper ions by electrosorption on modified activated carbon fiber with TiO2-coating. Chem. Ind. Eng. Process 30, 864–869 (2011)
S.Q.S. Yiping, Electrosorption removal ofCr(VI)on activated carbon modified by titania film. Chin. J. Environ. Eng. 1(2), 59–63 (2007)
M.W. Ryoo, G. Seo, Improvement in capacitive deionization function of activatedcarbon cloth by titania modification. Water Res. 37(7), 1527–1534 (2002)
Z.X. Wang, Phootcatayltic degradation of Azo dye over TiO2 supported on activated carbon, Master degree theses of master of Xian building university of science and technology, HAN Xuanli, 2006.03
H.-H. Li, H.-Y. Li, Effects of different pretreatment methods on structure and adsorption properties of activated carbon. Mater. Sci. Eng Powder Metall. 19(4), 647–653 (2014)
Y. Zhao, X. Hu, S. Shan, Renovated electroabsorptive desalination by using activated carbon coating electrodes. J. Saf. Environ. 3(32), 28–32 (2012)
K. E. Bouhidel, A. Lakehal. Infulence of voltage and flow rate on electrodeionization (EDI) process efficiency. Desalination 188(2), 411–421 (2006)
J. Li. Study on the preparation characterization and catalytic performance of the activated carbon—supported TIOZ catalyst. Master degree theses of master of the school of Chemical Engineering and Technology, Tianjin University, Tan xin, 2005.01
C. Ma, Y. Mao et al., Preparation and characterization of high specific area activated carbon by KOH/NaOH activation from abandoned capsicum straw. J. Funct. Mater. 44(5), 704–708 (2013)
H. Yang, M. Gong et al., Effect of pore size distribution on adsorption capacities of activated carbons for CH4 and CO2. Chin. J. Inorg. Chem. 27(6), 1053–1058 (2011)
S. Liu, X. Chen, Sol–Gel preparation and characterization of activated carbon supported TiO2 photocatalyst. Chin. J. Catal. 29(1), 19–24 (2008)
X.-Y. Chen, S.-X. Liu et al., Characterization and activity of TiO2/wAC composite photocatalyst prepared by acid catalyzed hydrolysis method. Wuli Huaxue Xuebao 22(5), 517–522 (2006)
F. Du, Q. Chen, Preparation and characterization of TiO2/activated carbon composite nano-fiber membrane. Mater. Rev. 25(10), 53–59 (2011)
H. Liu, N. Huang et al., Investigation on biocompatibility of biliary stent material. J. Funct. Mater. 6(42), 1071–1074 (2011)
H. Qing-Yu, W.Y.G. Ri, Z. Chun-Wang, Effects of the concentration of heavily oxygen vacancy of rutile TiO2 on electric conductivity performance from first principles study. Acta. Physica. Sinica. 16(62), 167201-1–167201-6 (2013)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Hh., Yang, J., Zhang, C. et al. The influence of the acidic scouring treatment on the wastewater treatment of TiO2 loaded activated carbon electrode. J Porous Mater 22, 887–895 (2015). https://doi.org/10.1007/s10934-015-9962-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-015-9962-4