Abstract
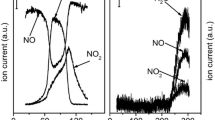
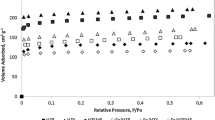
A series of iron-containing zeolites ZSM-5 with different iron loadings (0.5, 2 and 4 wt%) calcined at 900 °C and characterized by a high crystallinity were studied. The zeolites were tested in reactions of CO, methane, propylene and toluene oxidation by molecular oxygen in vacuum setup, as well as in flow reactor system using a model reaction mixture simulating automobile exhaust gases. Reactivities of components of the model reaction mixture change in the series: C3H6 > CO > C6H5CH3 > CH4. There were two stages of the hydrocarbon oxidation: they were oxidized first to CO and then reoxidized to CO2. Temperatures higher than 350 °C were required for methane oxidation to produce CO and CO2, any other products of methane partial oxidation being not found. High activity of Fe–ZSM-5 catalysts towards propylene and toluene oxidation was found to be determined by zeolite porous structure and capability to adsorb rapidly mentioned hydrocarbons.




Similar content being viewed by others
References
E.G. Derouane, J.C. Védrine, R. Ramos Pinto, P.M. Borges, L. Costa, M.A.N.D.A. Lemos, F. Lemos, F. Ramôa Ribeiro, The acidity of zeolites: concepts, measurements and relation to catalysis. Catal Rev 55, 454–515 (2013)
H.-X. Li, J.M. Donohue, W.E. Chrmier, Y.F. Chu, Application of zeolites as hydrocarbon traps in automotive emission controls. Stud. Surf. Sci. Catal. 58, 1375–1382 (2005)
I.V. Mishakov, A.A. Vedyagin, A.M. Volodin, M.S. Myakisheva, Adsorption catalytic neutralization of exhaust gases from diesel engines. Chem. Sustain. Dev. 19, 91–97 (2011)
B. Wichtelova, Z. Sobalik, J. Dedecek, Redox catalysis over metallo-zeolites contribution to environmental catalysis. Appl. Catal. B Environ. 41, 97–114 (2003)
A.A. Eliseev, A.S. Vyacheslavov, A.V. Lukashin, Yu.D. Tretyakov, I.P. Suzdalev, Yu.V. Maximov, P. Goernert, Iron-containing nanocomposites based on ZSM-5 zeolite. Int. J. Nanosci. 05, 459–463 (2006)
H.-Y. Chen, W.M.H. Sachtler, Activity and durability of Fe/ZSM-5 catalysts for lean burn NOx reduction in the presence of water vapor. Catal. Today 42, 73–83 (1998)
K.A. Dubkov, N.S. Ovanesyan, A.A. Shteinman, E.V. Starokon, G.I. Panov, Evolution of iron states and formation of α-sites upon activation of FeZSM-5 zeolites. J. Catal. 207, 341–352 (2002)
I. Yuranov, D.A. Bulushev, A. Renken, L. Kiwi-Minsker, Benzene hydroxylation over FeZSM-5 catalysts: Which Fe sites are active? J. Catal. 227, 138–147 (2004)
G.D. Pirngruber, P.K. Roy, R. Prins, The role of autoreduction and of oxygen mobility in N2O decomposition over Fe-ZSM-5. J. Catal. 246, 147–157 (2007)
J.B. Taboada, A.R. Overweg, P.J. Kooyman, I.W.C.E. Arends, G. Mul, Following the evolution of iron from framework to extra-framework positions in isomorphously substituted [Fe, Al]MFI with 57Fe Mössbauer spectroscopy. J. Catal. 231, 56–66 (2005)
G. Fierro, G. Moretti, G. Ferraris, G.B. Andreozzi, A Mössbauer and structural investigation of Fe-ZSM-5 catalysts: influence of Fe oxide nanoparticles size on the catalytic behaviour for the NO-SCR by C3H8. Appl. Catal. B Environ. 102, 215–223 (2011)
L.V. Pirutko, V.S. Chernyavsky, E.V. Starokon, A.A. Ivanov, A.S. Kharitonov, G.I. Panov, The role of α-sites in N2O decomposition over FeZSM-5. Comparison with the oxidation of benzene to phenol. Appl. Catal. B Environ. 91, 174–179 (2009)
G.I. Panov, Advances in oxidation catalysis; oxidation of benzene to phenol by nitrous oxide. Cattech 4, 18–31 (2000)
E.V. Starokon, M.V. Parfenov, S.S. Arzumanov, L.V. Pirutko, A.G. Stepanov, G.I. Panov, Oxidation of methane to methanol on the surface of FeZSM-5 zeolite. J. Catal. 300, 47–54 (2013)
M.V. Parfenov, E.V. Starokon, L.V. Pirutko, G.I. Panov, Quasicatalytic and catalytic oxidation of methane to methanol by nitrous oxide over FeZSM-5 zeolite. J. Catal. 318, 14–21 (2014)
H. Guesmi, D. Berthomieu, L. Kiwi-Minsker, Reactivity of oxygen species formed upon N2O dissociation over Fe–ZSM-5 zeolite: CO oxidation as a model. Catal. Commun. 11, 1026–1031 (2010)
M.A. Uddin, T. Komatsu, T. Yashima, Catalytic activity of framework iron in MFI-type ferrisilicate for the oxidation of carbon monoxide. Microporous Mater. 1, 201–205 (1993)
D.E. Doronkin, L.V. Piryutko, E.V. Starokon’, G.I. Panov, A.Y. Stakheev, Role of α-sites in the selective catalytic reduction of NOx with ammonia over Fe-ZSM-5 catalysts. Kinet. Catal. 53, 747–752 (2012)
M.S. Kumar, M. Schwidder, W. Grünert, A. Brückner, On the nature of different iron sites and their catalytic role in Fe-ZSM-5 DeNOx catalysts: new insights by a combined EPR and UV/VIS spectroscopic approach. J. Catal. 227, 384–397 (2004)
M. Iwasaki, H. Shinjoh, A comparative study of “standard”, “fast” and “NO2” SCR reactions over Fe/zeolite catalyst. Appl. Catal. A Gen. 390, 71–77 (2010)
G.I. Panov, G.A. Sheveleva, A.S. Kharitonov, V.N. Romannikov, L.A. Vostrikova, Oxidation of benzene to phenol by nitrous oxide over Fe-ZSM-5 zeolites. App. Catal. A Gen. 82, 31–36 (1992)
G.I. Panov, A.S. Kharitonov, V.I. Sobolev, Oxidative hydroxylation using dinitrogen monoxide: a possible route for organic synthesis over zeolites. Appl. Catal. A Gen. 98, 1–20 (1993)
B. Michalkiewicz, Partial oxidation of methane to formaldehyde and methanol using molecular oxygen over Fe-ZSM-5. Appl. Catal. A Gen. 277, 147–153 (2004)
V.V. Popovskii, Regularities of deep oxidation of substances over solid oxide catalysts. Kinet. Katal. 13, 1190–1203 (1972). (in Russian)
T.V. Andrushkevich, V.V. Popovskii, G.K. Boreskov, Catalytic properties of oxides of metals from IV period of periodic table with respect to oxidative reactions. I. Methane oxidation. Kinet. Katal. 6, 860–863 (1965). (in Russian)
G.I. Panov, E.V. Starokon, L.V. Pirutko, E.A. Paukshtis, V.N. Parmon, New reaction of anion radicals O− with water on the surface of FeZSM-5. J. Catal. 254, 110–120 (2008)
G.I. Panov, K.A. Dubkov, E.V. Starokon, Active oxygen in selective oxidation catalysis. Catal. Today 117, 148–155 (2006)
Acknowledgments
This work was supported by Russian Academy of Sciences (Project #V.45.3.2). The authors are grateful to V. V. Mokrinskii and T. A. Komnik for their assistance in catalyst testing.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Starokon, E.V., Vedyagin, A.A., Pirutko, L.V. et al. Oxidation of CO and hydrocarbons with molecular oxygen over Fe–ZSM-5 zeolite. J Porous Mater 22, 521–527 (2015). https://doi.org/10.1007/s10934-015-9922-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-015-9922-z